Article Text
Abstract
Introduction and objective The role of secreted gut microbial components in the initiation of systemic inflammation and consequences of antibiotic therapies on this inflammatory process are poorly elucidated. We investigate whether peripheral innate cells mount an inflammatory response to gut microbial components, the immune cells that are the primary drivers of systemic inflammation, the bacterial populations that are predominantly responsible, and whether perioperative antibiotics affect these processes.
Method and experimental design Conditioned supernatants from gut microbes were used to stimulate murine innate cell types in vitro and in vivo, and proinflammatory responses were characterised. Effects of antibiotic therapies on these responses were investigated using a model of experimental intestinal barrier damage induced by dextran sodium sulfate.
Results Proinflammatory responses in the periphery are generated by components of anaerobes from the Bacteroidetes phylotype and these responses are primarily produced by myeloid dendritic cells. We found that the common prophylactic therapy for sepsis (oral neomycin and metronidazole administered to patients the day prior to surgery) is ineffective for clearing Bacteroidetes from the murine intestine. A point of critical consequence of this result is the increased systemic inflammation and premature death observed in treated mice, and these outcomes appear to be independent of gut bacterial spread in the initial phase of intestinal barrier damage. Importantly, spillage of gut microbial products, rather than dissemination of gut microbes, may underlay the initiation of systemic inflammation leading to death.
Conclusions Our data further affirm the importance of a balanced gut microflora biodiversity in host immune homeostasis and reinforce the notion that inadequate antibiotic therapy can have detrimental effects on overall immune system.
- ANTIBIOTIC THERAPY
- INTESTINAL BACTERIA
- SEPSIS
- IMMUNE RESPONSE
- INFLAMMATION
This is an Open Access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Statistics from Altmetric.com
Summary box
What is already known about this subject?
▸ Diversity of gut microbiota is a vital part of intestinal defence, and once this is disturbed, the intestine is vulnerable to the detrimental effects of gut bacteria.
▸ Epidemiological data suggest that sepsis now represents a rising worldwide healthcare burden. Initial studies focused attention on bacterial translocation across the intestinal mucosa into the systemic milieu as a unifying mechanism to explain sepsis.
▸ More than half of patients who developed sepsis did not have an identifiable bacterial infection, and current studies could not fully explain this phenomenon.
What are the new findings?
▸ Components of cultivable anaerobes from the Bacteroidetes phylotype are the primarily inducers of proinflammatory responses and these responses are primarily produced by myeloid dendritic cells.
▸ The common prophylactic therapy for sepsis which consists of neomycin and metronidazole given orally to patients the day before surgery is ineffective in clearing Bacteroidetes from the murine intestine.
▸ In response to acute intestinal injury, this prophylactic therapy induced increased systemic inflammation and premature death when compared with treatment-free mice, and this outcome is independent of the dissemination of cultivable anaerobes.
▸ Intraperitoneal spread of gut-derived anaerobe of antibiotic-treated mice showed declined survival percentage compared with that with untreated mice, indicating that spillage of gut microbial products, rather than dissemination of gut microbes themselves, may underline the initiation of systemic inflammation leading to death in the early phase of intestinal barrier injury.
How might it impact on clinical practice in the foreseeable future?
▸ How to bridge and extrapolate findings from mouse models to the setting of human sepsis is always a daunting challenge. Nevertheless, based on our data it would appear inadvisable to prescribe the perioperative therapy of neomycin and metronidazole to leaky gut-prone individuals.
▸ Novel treatments that will specifically target anaerobes from the Bacteroidetes could provide a successful infection-control approach for the treatment of systemic inflammation leading to sepsis and death.
▸ Therapeutic tools that block the activation of myeloid dendritic cells could be beneficial to treat sepsis.
Introduction and rationale
The gut microbiota is engaged in a dynamic interaction with the immune system, affecting different aspects of its development and function.1–4 The mechanisms through which the microbiota exerts its immune influences remain largely undefined, but include the recognition of secreted bacterial products by immune cells and elaboration of signalling molecules.3 ,5 The overall balance in the composition of the gut microbial communities is important in ensuring homeostasis or lack thereof in the gut and systemic sites.1 ,3 ,6 Perhaps, the most serious manifestation of a failure in the mammalian intestinal microbial symbiosis is the clinical state of systemic inflammation leading to sepsis. Translocation of gut bacteria, which are normally retained within the lumen of the intestinal tract across the intestinal barrier, is thought to be integral to this clinical process.7 ,8 However, more than half of patients who developed sepsis do not show evidence of bacterial contamination in the periphery,8–10 and there are no published studies that clearly explain this phenomenon.
Most of the effects of intestinally derived bacteria on systemic immune cells are mediated by a controlled passage of bacterial products that act as immunomodulators.11 ,12 The advantage of these molecules over the bacteria themselves is that they can cross the intestinal epithelium and reach peripheral sites relatively easily. Thus, one possibility during the initial phase of sepsis is that uncontrolled spillage of secreted bacterial products, rather than gut bacteria themselves, may occur causing the initiation of systemic inflammatory responses. Therefore, we sought to understand the consequences of the interaction between secreted gut microbial components and systemic immune cells. Specifically, we asked whether systemic innate cells can mount an inflammatory response to secreted gut microbial components, which immune cells were the primary drivers of inflammation, and which bacterial populations were predominantly responsible. We show that proinflammatory responses in the periphery are generated by gut microbial components of the Bacteroidetes phylotype and these responses are primarily produced by CD11b+ CD11c+ dendritic cells (DCs).
It has been argued that aggressive perioperative infection-control strategies such as the use of preoperative antibiotics directly upregulate signalling and cellular-microbial cross-talk pathways within the gut with deleterious consequences for the host, such as microbial drug resistance and persistent rates of postoperative sepsis.7 This theory is reinforced by recent clinical studies which have demonstrated that surgical antibiotic use increases the risk of Clostridium difficile infection.13 Based on these observations, we tested the efficacy of preventive treatment for bowel surgery in clearing gut Bacteroidetes. A common oral antibiotic regimen is the Nichols-Condon bowel preparation and consists of neomycin and erythromycin administered the day prior to surgery.14 Metronidazole has been recently substituted for the erythromycin due to its seemingly increased efficacy against anaerobic microorganisms in the gut.15 ,16 Using doses similar to those prescribed to individual patients, we find that this prophylactic therapy, although is efficient for clearing most gut anaerobes, is ineffective for clearing those of the Bacteroidetes phylotype from the murine intestine. Moreover, we find that prophylactic treatment followed by intestinal barrier damage induced more rapid death kinetic compared with antibiotic-free mice. Together, our data further affirm the importance of a balanced gut microflora biodiversity in host immune homeostasis and suggest that prescribing oral antibiotics to patients, especially those with a leaky gut syndrome, have to be carefully weighed if outcomes from postoperative systemic inflammation leading to sepsis are to be improved.
Materials and methods
Mice
C57BL/6 and BALB/c mice were purchased from Taconic (Germantown, NY). All mice were maintained in a specific pathogen-free barrier unit at Rutgers University and were used between 6 and 12 weeks of age.
Ethics and dissemination
All experiments followed guidelines of Rutgers University Institutional Animal Care and Use Committee (IACUC). Approval for use of mice was obtained from Rutgers University according to criteria outlined in the Guide for the Care and Use of Laboratory Animals from the National Institutes of Health.
Bacterial cultures
The experimental setup is described in detail in the online supplementary methods.
Assessment of anaerobes in the gut and extraintestinal tissues
The experimental setup is described in detail in the online supplementary methods.
Isolation of splenocytes and stimulation with bacterial-conditioned supernatants
The experimental setup is described in detail in the online supplementary methods.
Flow cytometry
The experimental setup is described in detail in the online supplementary methods.
DNA isolation from bacteria
The experimental setup is described in detail in the online supplementary methods.
Quantitative PCR
The experimental setup is described in detail in the online supplementary methods.
Treatment of mice with antibiotics
The experimental setup is described in detail in the online supplementary methods.
Systemic injection of bacterial-conditioned supernatants
The experimental setup is described in detail in the online supplementary methods.
Mouse model of intestinal injury induced by dextran sodium sulfate
The experimental setup is described in detail in the online supplementary methods.
Blood analysis
The experimental setup is described in detail in the online supplementary methods.
Survival assays
We used time to death as well as moribund for all survival assays. We identified moribund mice as those mice that are unable to move when gently touched or unable to self-correct when placed on their side (ataxia). Moribund mice were euthanised in compliance with IACUC protocols at the Rutgers University.
Statistics
GraphPad Prism software (La Jolla, California, USA) was used to conduct unpaired, two-tailed Student t test for sample analysis. Results with p<0.05 were considered significant.
Results
Conditioned supernatants derived from gut anaerobes induce proinflammatory responses from specific antigen-presenting cell subsets
The importance of innate cells as the first-line of defence against microorganisms and their microbial products is undisputable. However, while the impact of gut microbial products on gut innate cells is increasingly understood, the consequences of interaction between these components and distant peripheral innate cells remain unclear.
The use of 16S ribosomal RNA (rRNA) gene sequences has been by far the most common methodology to study gut bacterial phylogeny and taxonomy.17 However, whereas this method can be used for a number of bacterial identification purposes, the procedures that enable the acquisition of secreted bacterial components are mainly confined to the use of bacterial culture systems. Therefore, to address our question, we first cultured luminal contents of the small intestine and colon from C57BL/6 mice under aerobic and anaerobic conditions as described in Methods section,18–22 and conditioned supernatants were used to simulate splenocytes isolated from C57BL/6 mice. Four hours later, antigen-presenting cells (APC) were examined and production of proinflammatory cytokines was assessed in response to microbial products derived from cultivable aerobic and anaerobic supernatants (figure 1). We found that supernatants of anaerobes cultured from the small intestine and colon induced substantially tumour necrosis factor (TNF) α and pro-interleukin (IL) 1β than aerobic supernatants (figure 1A, B, and see online supplementary figure S1). Interestingly, the strong TNF-α response was found to be specific to CD11b+ CD11c+ DCs as neither CD11b− CD11c+ DCs nor CD11b+ CD11c− F4/80+ macrophages expressed more TNF-α in response to anaerobic than aerobic supernatants (figure 1A). Likewise, CD11b+ CD11c+ DCs expressed significantly more pro-IL-1β when exposed to conditioned medium from cultivable anaerobes than aerobes of the colon (see online supplementary figure S1). This suggests that proinflammatory responses generated by cultivable bacterial components are primarily induced by gut anaerobes and selectively produced by CD11b+ CD11c+ myeloid DC subset. Of note, the natural ecosystem of the gut microbiota is very complex and culturing luminal faecal contents does not enable the growth of all gut bacteria in culture systems since most species are not cultivable.17 This is not a pitfall of the experimental strategy but rather a limitation of current methodologies for gut bacterial culture procedures.
Proinflammatory responses are induced by components of gut anaerobes from the Bacteroidetes and produced by myeloid dendritic cells (DCs). (A) Bar graphs showing tumour necrosis factor (TNF) α expression as a % of CD11b+ CD11c+ DCs, CD11b− CD11c+ DCs, and CD11b+ CD11c− F4/80+ (n=4). (B) Dot plots showing expression of TNF-α by CD11b+ CD11c+ DCs and CD11b− CD11c+ DCs. Numbers indicate the % TNF-α cells. (C) Bar graphs showing that Firmicutes dominate the aerobic cultures and a large portion of aerobic Firmicutes were Enterococcus (n=4). (D) Bar graph showing that anaerobic cultures of small intestine and colon bacteria have relatively high proportions of Bacteroidetes (n=4–6). (E–G) Mice were gavaged with PBS (untreated) or a combination of neomycin and metronidazole (Neo/Met) used as prophylaxis for bowel surgery for 24 h, or injected intravenously with a combination of piperacillin and tazobactam (Pip/Taz) used as a chemotherapy for sepsis, every 24 h for three days. (E) Bar graph showing the relative number of cultivable anaerobes in antibiotic-treated mice to antibiotic-free animals (n=5–8). (F) Bar graph showing ratio of Bacteroidetes to Firmicutes in untreated and antibiotic-treated mice (n=4). (G) Bar graph showing the proportion of total gut bacteria represented by Bacteroidetes (n=4). *p<0.05, **p<0.005, ***p<0.0005 using two-tailed Student t test. SSC, side scatter.
Aerobic and anaerobic bacterial cultures from the small intestine and colon are dominated by distinct phyla
The gut of mammals is dominated by two major phylotypes of bacteria: Firmicutes and Bacteroidetes.23 ,24 Maintenance of a prescribed equilibrium between these two major groups is critical, as shifts in the balance between these two groups has been associated with obesity, irritable bowel syndrome, Crohn's disease, ulcerative colitis and colon cancer.24–29 Since products derived from cultivable anaerobes but not aerobes were potent inducer of proinflammatory cytokines by peripheral APCs, we next characterised their composition of Firmicutes and Bacteroidetes. To this end, DNA was isolated from both bacterial sources used to generate our conditioned bacterial supernatants and the relative proportions of Firmicutes versus Bacteroidetes was determined by quantitative PCR (QPCR) using specific primers for 16S rRNA gene.30 We found that Firmicutes dominate the aerobic cultures, with significantly more Firmicutes found in the cultures of aerobes compared with their anaerobic counterparts (figure 1C). To further extend this finding, we next examined the relative proportion of a major genus of Firmicutes in the gut, namely Enterococcus.31 We found that a large portion of aerobic Firmicutes cultured from the small intestine and colon were Enterococcus and that there was significantly more Enterococcus in the aerobic cultures compared with anaerobic counterparts (figure 1C). However, unlike aerobic cultures, anaerobic cultures from small intestine and colon revealed significantly higher proportions of Bacteroidetes spp. (figure 1D). Consistent with different effects in driving proinflammatory responses from peripheral APCs, these results suggest that aerobic and anaerobic cultures from small intestine and colon are dominated by distinct phyla, with aerobic cultures dominated by Firmicutes and anaerobic cultures mostly represented by Bacteroidetes.
Changes of Bacteroidetes:Firmicutes ratios may dictate efficacy of antibiotic treatment for sepsis
Our results suggest that secreted microbial components of cultivable anaerobes derived from the Bacteroidetes are potent inducers of proinflammatory cytokines by peripheral APCs. We next sought to understand whether Bacteroidetes could play a role in the induction of systemic inflammation leading to sepsis and death. To address this question, we first treated mice orally with a combination of neomycin and metronidazole (Neo/Met) used as antibiotic prophylaxis for bowel surgery32–34 and examined by QPCR the efficacy of this prophylactic therapy in clearing Bacteroidetes from the murine intestine (figure 1E, F). As expected, we found that numbers of cultivable anaerobes decreased significantly (∼70%) in antibiotic-treated mice compared with untreated littermates (figure 1E). However, while the total number of cultivable anaerobes decreased in response to antibiotic treatment, the ratio of Bacteroidetes to Firmicutes in the anaerobes that survived this treatment revealed a substantially increased (∼30-fold) proportion of Bacteroidetes (figure 1F). Moreover, when examining the proportion of total gut bacteria represented by Bacteroidetes, we found no decrease as a result of Neo/Met treatment (figure 1G), suggesting that therapy with Neo/Met is efficient at clearing most cultivable anaerobes but not Bacteroidetes spp.
To further extend this observation, we next asked whether a treatment for intra-abdominal infections following bowel surgery could be more effective at clearing gut Bacteroidetes. To this end, mice were treated with piperacillin/tazobactam (Pip/Taz) intravenously for 3 days and efficacy of this treatment in clearing Bacteroidetes from the intestine was examined by QPCR as previously described.30 ,35 Unlike treatment with Neo/Met, treatment with Pip/Taz significantly shifted the ratio of Bacteroidetes:Firmicutes in favour of Firmicutes (figure 1F) and drastically reduced both the number and percentage of Bacteroidetes in the gut to <3% of the total bacterial species (figure 1E, G). Thus, while the preventive treatment with Neo/Met was ineffective in clearing Bacteroidetes from the murine intestine, a more effective treatment with Pip/Taz was highly efficient at Bacteroidetes clearance. Together, these data indicate that efficacy of antibiotic treatment as a control strategy for systemic inflammation leading to sepsis may correlate with clearance of Bacteroidetes from the gut.
Prophylactic treatment increases systemic inflammation and causes more rapid death in response to intestinal barrier damage
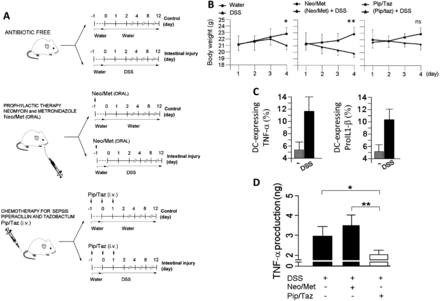
Since cultivable anaerobes formed by the Bacteroidetes are potent inducers of proinflammatory responses by peripheral APCs and the prophylactic Neo/Met treatment is ineffective at clearing these anaerobes (figure 1), we hypothesised that the preventive antibiotic treatment (the Nichols-Condon bowel preparation) may be detrimental rather than beneficial at reducing risks of systemic inflammation leading to sepsis and death in conditions of intestinal barrier damage. Caecal ligation and puncture has been proposed as a surgical murine model to study the pathogenesis of sepsis.36 However, because this model utilises the surgical perforation of the ligated caecum which results in immediate and consistent drainage of caecal bacteria into the peritoneal cavity,36 its applicability was unsuitable for this study since our intent is to understand the role of secreted bacterial products rather the bacteria themselves during the initial phase of intestinal barrier injury. Therefore, to test our hypothesis, we used a minimally invasive sepsis mouse model that couples antibiotic treatments with dextran sulfate sodium (DSS) induced intestinal barrier damage.37 DSS is toxic to colonic epithelial cells and typically causes a colitis-like disease in BALB-c mice characterised by weight loss and intestinal barrier injury.38 Briefly, BALB-c mice were treated with drinking water only (control) or drinking water supplemented with DSS continuously for 4–12 days (figure 2A, upper panel). By the end of the fourth day, significant physical and immunological changes were observed between both mouse groups (figure 2B first panel, C). DSS-treated BALB-c mice lost significant body weight compared with untreated controls (figure 2B first panel). In addition, percentages of DC-expressing proinflammatory TNF-α and pro-IL-1β were higher in the spleen of DSS-treated mice compared with untreated animals (figure 2C), suggesting the initiation of systemic inflammatory responses at day 4 post-DSS treatment. Next, we asked whether Nichols-Condon bowel preparation can further accelerate or rather attenuate this immune process. To this end, BALB-c mice were treated orally with Neo/Met; 24 h later, animals were provided with sterile drinking water (control) or drinking water supplemented with DSS continuously for 4 days (figure 2A, middle panel). In parallel experiments, mice were treated with Pip/Taz intravenously every 24 h for 3 days. At the end of the first day, mice were given autoclaved drinking water (control) or drinking water supplemented with DSS continuously for 4 days (figure 2A, lower panel). The goal of these experiments was to compare the outcomes of ineffective clearance of Bacteroidetes (Neo/Met) versus effective clearance of Bacteroidetes (Pip/Taz) in response to acute intestinal barrier damage. Consistent with previous results, the use of the prophylactic therapy induced significant weight loss in response to DSS compared with other animal groups (figure 2B). Plasma concentration of TNF-α is frequently elevated in septic patients and mouse models of sepsis and correlates with severity and outcome.39 ,40 In this context, our data revealed that plasma concentration of TNF-α did not decrease as a result of preventive therapy (figure 2D). Furthermore, mice treated with the prophylactic therapy died more rapidly in response to DSS treatment compared with wild type littermates that received DSS only or DSS in combination with the Pip/Taz therapy (figure 3A). Therefore, Neo/Met treatment used as prophylaxis for sepsis appears to be rather inefficient at reducing systemic inflammation and may even accelerate the initiation of this process resulting in increased mortality in intestinally-injured mice.
Neo/Met therapy is inefficient for reducing systemic inflammation and causes faster death in intestinally-injured mice. (A) Mouse model of intestinal injury was developed using 6–8 week old BALB-c mice provided with drinking water only (control) or supplemented with DSS continuously for the duration of the experiments. Mice were orally injected with the Nichols-Condon bowel preparation which consists of a combination of (Neo/Met, 85 and 145 mg/kg, respectively). Twenty-four hours later, animals were transferred to new cages and provided with autoclaved drinking water (control) or drinking water supplemented with DSS (w/v) continuously for 4–12 days (Middle panel). Other mice were injected intravenously with Pip/Taz (1500 and 187.5 mg/kg, respectively) every 24 h for three days. At the end of the third day, mice were transferred to new cages and were given autoclaved drinking water (control) or drinking water supplemented with DSS continuously for 4–12 days (lower panel). (B) Weight loss of mice treated with PBS or antibiotics only (n=4), or DSS only (n=10), or DSS in combination with antibiotic therapies (n=10) for 4 days. (C) Bar graphs showing TNF-α and PROIL1-β production by splenic DCs in mice untreated (n=4) and DSS-treated animals (n=10). (D) Serum concentrations of TNF-α at day 4 post-DSS treatment. Bar graphs showing relative production of TNF-α in the blood (n=4–10). NS (p>0.01), *p<0.05, **p<0.005, using two-tailed Student t test (DCs, dendritic cells; DSS, dextran sulfate sodium; i.v., intravenously; Neo/Met, neomycin and metronidazole; NS, not significant; Pip/Taz, piperacillin and tazobactam; PROIL1-β, prointerleukin 1β; TNF-α, tumour necrosis factor α).
Spread of cultivable anaerobes occurs only at a later time point of intestinal injury and further accelerates death in Neo/Met-treated mice. (A) Survival of mice that received water or antibiotic only (n=4) or water supplemented with DSS (n=10), or DSS in combination with antibiotic therapies (n=20) for 4 days. (B) Amounts of cultivable anaerobes at day 4 post-DSS treatment in the spleen and liver of antibiotic-free and Neo/Met-treated mice (3 independent experiments of n=3). (C) Survival of mice that were injected i.p. with anaerobic broth (vehicle, n=5) or supernatants of cultivable anaerobes from the colon of untreated (n=10) or Neo/Met-treated (n=10) mice. (D) Amounts of cultivable anaerobes at day 12 post-DSS treatment in the spleen and liver of antibiotic-free and antibiotic-treated mice (3 independent experiments of n=3). (E) Survival of mice that received water or antibiotics only (n=4) or water supplemented with DSS (n=20), or DSS in combination with Neo/Met (n=20) for 12 days. NS (p>0.01), *p<0.05, using two-tailed Student t test (DSS, dextran sulfate sodium; i.p, intraperitoneal; Neo/Met, neomycin and metronidazole; NS, not significant; Pip/Taz, piperacillin/tazobactam). CFU, colony-forming unit; PBS, phosphate buffered saline.
Intraperitoneal spread of gut anaerobe products prepared from colonic contents of Neo/Met-treated mice induces rapid death in host mice
Translocation of bacteria, particularly anaerobes, across the intestinal mucosa into the systemic milieu was proposed as a unifying mechanism to explain early postoperative sepsis.7 ,10 ,41 To determine whether faster moribundus or death induced by the preventive therapy is caused by systemic dissemination of gut-derived microbes, we assessed the number of anaerobes in extraintestinal organs at day 4 post-DSS treatment. Our data showed no significant levels of cultivable anaerobes in the spleen and liver of Neo/Met-treated mice compared with untreated controls (figure 3B). These findings, however, do not exclude the possibility of the development of non-cultivable species in these tissues or the development of bacteraemia in the portal circulation that could have been cleared in the liver prior to the initiation of systemic inflammation. Nevertheless, these outcomes suggest that negative cultures in the spleen and liver are associated with a lack of significant level of cultivable anaerobes in these tissues in the early phase of intestinal injury. Since cultivable anaerobe products were potent inducers of proinflammatory cytokines by peripheral APCs (figure 1); we hypothesised that spread of gut anaerobe products may be an aetiological agent inducing the negative outcomes resulting from the prophylactic therapy. To test this hypothesis, we performed experiments to mimic the spread of gut-derived cultivable anaerobe products in extraintestinal organs (figure 3C). Briefly, healthy wild-type mice were injected intraperitoneally with bacteria-free supernatants prepared from cultivable colonic contents of untreated or Neo/Met-treated animals and outcomes from such treatment were assessed in vivo. Unlike injection of bacterial material from antibiotic-free mice, conditioned supernatants from Neo/Met-pretreated mice induced high mortality after only 48 h of treatment (figure 3C), indicating that early death induced by the prophylactic Neo/Met may be caused by the spread of gut anaerobe products, rather than the microbes themselves, in the initial phase of intestinal barrier damage.
Prophylactic treatment for sepsis induces increased dissemination of gut anaerobes in response to chronic, but not acute, intestinal barrier injury
During early intestinal barrier damage, we observed no detectable levels of cultivable anaerobes in the liver and spleen of untreated and antibiotic-treated mice (figure 3B). However, we could not eliminate the possibility that translocation of gut anaerobes across the intestinal mucosa into the systemic milieu may occur at later time points of intestinal injury. To test this possibility, BALB-c mice were treated with DSS to induce intestinal injury, coupled with Neo/Met therapy, for 12 days. Unlike in the early phase of intestinal injury, analysis at a later time point revealed the detection of gut cultivable anaerobes in the spleen and liver of treated mice (figure 3D), and this phenotype correlated with increased death rate in antibiotic-treated animals (figure 3E).
All together, the prophylactic therapy with Neo/Met followed by acute intestinal barrier damage appears to induce more rapid death in mice that may be caused by the spread of gut anaerobe products, rather than the anaerobes themselves. However, the dissemination of gut anaerobes occurs at later time points of intestinal barrier damage and this may further accelerate death.
Discussion
The intestinal tract houses the largest population of bacteria in the body and the majority of these microorganisms are anaerobes; of these ∼25% are species of Bacteroidetes.41 ,42 These bacteria maintain a complex and generally beneficial relationship with the host when retained in the gut.1 ,3 However, when Bacteroidetes species escape the gut, usually resulting from disruption of the intestinal barrier, they can cause significant pathology, including inflammation and abscess formation.41 ,43 ,44 In this context, our findings suggest the role of anaerobes from the Bacteroidetes phylotype are important inducers of systemic inflammation and underscore the importance of an overlooked aspect of these gut bacteria: their products in the induction of systemic inflammation leading to death.
Sepsis with its complications is still a major challenge in contemporary medicine. Depending on the standards of medical care, the worldwide mortality rates in septic humans range from 30% to 70% (with an aggregate mortality rate of ∼50%).45 ,46 Despite more than 20 years of extensive research and development of numerous therapeutic approaches used in clinical settings, the incidence of sepsis and the number of sepsis-related deaths are rising at rates between 1.5% and 8% per year.45 ,46 For a long time, it was believed that sepsis was caused by an overwhelming immune response of the patient to invading microorganisms.14 ,47 As a precautionary measure, antibiotic regimens are given to patients before and/or during surgery.14 ,47 A common oral antibiotic regimen is the Nichols-Condon bowel preparation, which consists of neomycin and erythromycin given the day before surgery.14 ,32 Metronidazole was recently substituted for the erythromycin because of its seemingly increased efficacy against anaerobic microorganisms in the gut.48 A major outcome from our study is that secreted microbial components of cultivable Bacteroidetes are potent inducers of proinflammatory cytokines by peripheral DCs. Based on this finding; we tested the efficacy of the preventive therapy for bowel surgery in clearing Bacteroidetes and compared its efficacy to Pip/Taz treatment for intra-abdominal infection following bowel sepsis.49 ,50 While treatment for sepsis was highly efficient at Bacteroidetes clearance, we found that oral therapy with Neo/Met was ineffective for clearing Bacteroidetes from the murine intestine. One point of crucial consequences from this result was increased systemic inflammation and more rapid time death in mice treated with the prophylactic Neo/Met in condition coupled with intestinal barrier damage induced by DSS.
Still, the scope of this study is that the interplay between intestinally derived microbial products and the host may determine the outcome after intestinal barrier damage. Spillage of gut microbial products outside the intestinal mucosa may generate several possible scenarios (see online supplementary figure S3). Gut microbial products that do not derive from Bacteroidetes, such as aerobe Firmicutes, may be less harmful, in which case weak inflammatory responses are developed. As an example, bacterial products of Enterococcus faecalis, a representative of gut Firmicutes,31 had little to no effect on driving proinflammatory responses in vitro (see online supplementary figure S2). However, when inflammation is established by Bacteroidetes products, potent inflammatory responses may follow. These responses can be regulated by a network of proinflammatory cytokines including TNF-α and pro-IL-1β derived primarily from myeloid DCs. This initial response may determine the systemic expansion of the inflammatory response. Moreover, because Bacteroidetes products contain potent proteolytic enzymes of the gut epithelium such as the Bacteroides fragilis enterotoxin,41 they may at later stages, work in concert with other processes to increase the enzymatic destruction of mucosal barriers and facilitate the dissemination of gut microbes within the host leading to an immune collapse and faster death.
Cross-talks between the host and secreted bacterial products from intestinal microbiota are poorly understood, and we do not know yet to what extent the encounters between these secreted microbial products and the host can be modulated. In many settings the most cost-effective way to improve post intestinal barrier damage outcomes is to strengthen the quality of antibiotic treatments. However, in many parts of the world the spread of multidrug-resistant bacteria seriously threatens the success of antibiotic therapies.51 However because eliminating the need for antibiotics is unrealistic at this time, a better understanding of how antibiotic therapies impact the gut microbiota and host immunity is needed, so that strategies to mitigate the negative effects can be developed. How to bridge and extrapolate findings from mouse sepsis models to the setting of human sepsis is always a daunting challenge. Nevertheless, based on these findings, it would appear inadvisable to prescribe the perioperative therapy of Neo/Met to leaky gut-prone individuals.
Acknowledgments
The authors thank Drs Yasmina Laouar and Yufang Shi for critical reading of the manuscript, and Mariel Watkins for technical assistance with faecal DNA isolation and QPCR.
References
Footnotes
Contributors DS, AE and MT performed the experiments and analysed the data. DS and AL designed the experiments and analysed the data. AL wrote the manuscript.
Funding This work is supported by the Robert Wood Johnson Foundation (grant # 67038) to the Child Health Institute of New Jersey.
Competing interests None.
Provenance and peer review Not commissioned; externally peer reviewed.
Data sharing statement No additional data are available.