Article Text
Abstract
BACKGROUND AND AIMS Controversy surrounds the risk of colorectal cancer (CRC) in ulcerative colitis (UC). Many studies have investigated this risk and reported widely varying rates.
METHODS A literature search using Medline with the explosion of references identified 194 studies. Of these, 116 met our inclusion criteria from which the number of patients and cancers detected could be extracted. Overall pooled estimates, with 95% confidence intervals (CI), of cancer prevalence and incidence were obtained using a random effects model on either the log odds or log incidence scale, as appropriate.
RESULTS The overall prevalence of CRC in any UC patient, based on 116 studies, was estimated to be 3.7% (95% CI 3.2–4.2%). Of the 116 studies, 41 reported colitis duration. From these the overall incidence rate was 3/1000 person years duration (pyd), (95% CI 2/1000 to 4/1000). The overall incidence rate for any child was 6/1000 pyd (95% CI 3/1000 to 13/1000). Of the 41 studies, 19 reported results stratified into 10 year intervals of disease duration. For the first 10 years the incidence rate was 2/1000 pyd (95% CI 1/1000 to 2/1000), for the second decade the incidence rate was estimated to be 7/1000 pyd (95% CI 4/1000 to 12/1000), and in the third decade the incidence rate was 12/1000 pyd (95% CI 7/1000 to 19/1000). These incidence rates corresponded to cumulative probabilities of 2% by 10 years, 8% by 20 years, and 18% by 30 years. The worldwide cancer incidence rates varied geographically, being 5/1000 pyd in the USA, 4/1000 pyd in the UK, and 2/1000 pyd in Scandinavia and other countries. Over time the cancer risk has increased since 1955 but this finding was not significant (p=0.8).
CONCLUSIONS Using new meta-analysis techniques we determined the risk of CRC in UC by decade of disease and defined the risk in pancolitics and children. We found a non-significant increase in risk over time and estimated how risk varies with geography.
- ulcerative colitis
- colorectal cancer
- risk
- meta-analysis
Abbreviations used in this paper
- CRC
- colorectal cancer
- UC
- ulcerative colitis
- pyd
- person years duration
Statistics from Altmetric.com
Colorectal cancer (CRC) was first recognised as a complication of ulcerative colitis (UC) by Crohn and Rosenberg in 19251and since then a multitude of epidemiological studies have confirmed this increased risk. The exact magnitude of the risk remains a subject of controversy because of various biases and methodological errors in published studies.2-6 Although CRC in UC only accounts for 1% of all cases of CRC seen in the general population,7 it is a serious sequel of the disease and accounts for one sixth of all deaths in UC patients.8 As a result it deserves our attention.
Early estimates of CRC complicating UC were based on crude percentages and all were from major medical institutions, predominantly tertiary referral centres. These centres saw a greater proportion of patients who had more severe recalcitrant disease and also patients who had been referred with a diagnosis of cancer. These series were based on patients admitted to hospital and risks were related to the hospital population rather than the larger population of the host community. These and other factors led to an initial over reporting of the cancer risk. Later population based studies covered defined geographical areas and aimed for complete case ascertainment. These studies are superior with respect to methodological standards and lean to more conservative risk estimates. However, population based series probably include more patients with limited disease and therefore may underestimate the risk.
There is a general consensus from all studies that the CRC risk is highest in those with extensive disease of long duration. There is less certainty concerning how the risk may vary with geographical location. The worldwide variations reported may represent true differences relating to genetic or environmental factors. However, again the methods employed were not uniform and consequently it is not surprising that the CRC risk has been reported to be as low as 1.4% at 18 years9 and as high as 34% after 25 years of disease.10
The aim of this paper is to give an overall estimate of the risk in all patients with UC by decade, define the risk for children and those with extensive colitis, and give CRC incidence rates by country where possible.
Method
The meta-analysis was conducted according to the guidelines produced by the NHS Centre for Reviews and Dissemination at York University.11
IDENTIFICATION OF PRIMARY STUDIES
All published reports citing the risk of CRC in UC were collected by conducting a literature search on Medline using the following keywords: colorectal cancer, ulcerative colitis, surveillance studies, dysplasia, risk factors, and children. A comprehensive search of reference lists of all review articles and of the retrieved original studies was performed to find studies not identified by the Medline search. This identified 194 independent studies dating back to 1925.
INCLUSION AND EXCLUSION CRITERIA
English language articles were included where there was a clear definition of the population of patients being studied and where the criteria for diagnosing UC and CRC, together with their outcomes, were well described. Studies citing cancer mortality statistics (not cancer incidence) were excluded as this is not a true representation of cancer incidence. Duplicate publications and studies that obviously combined patients with UC and Crohn's disease in a common analysis were also excluded.
DATA EXTRACTION
One author (JE) read each paper and extracted several study and patient population characteristics using a predefined review form (see appendix). Studies that were suitable for inclusion (from which a minimum data set of number of patients and number of cancers detected could be extracted) were placed in to one of three categories:
- (1)
- Crude cancer prevalence only reported.
- (2)
- Cancer incidence and duration of patient follow up reported.
- (3)
- Cancer incidence stratified by decade and duration of patient follow up reported.
STATISTICAL ANALYSIS
All analyses were performed using Stata and macros (meta-analysis programs) for conducting meta-analyses.12-14 Overall pooled estimates, together with 95% confidence intervals (CI), of the prevalence and incidence of CRC were obtained using a random effects model on either the log odds or log incidence scale, as appropriate.15 Changes in the log incidence rate over time were assessed using mixed effects meta-regression techniques.16 The size of the circles in fig 2 are inversely proportional to the variance associated with the estimate of the log incidence rate in each study and the regression line was estimated using mixed effects meta-regression techniques. Where possible the actual observed number of cases of CRC and the person years duration (pyd) of follow up were extracted from papers. When only the number of cases of CRC and cumulative probabilities were reported, pyd was calculated.17 In addition to estimating the magnitude of the CRC risk in UC, and how that risk varies temporally, subgroup analyses were performed to explore between study heterogeneity.
Temporal relationship of colorectal cancer, overall and by geographical location.
Results
A total of 194 studies were identified. Of these, five reported cancer mortality data,8 ,18-21 10 did not give details concerning the background population,22-31 two included patients with Crohn's disease,32 ,33 four were reviews only,34-37 26 were updated by subsequent studies,9 ,38-62 and 31 overlapped with other studies or included the same patients.59 ,63-92 This left 116 studies suitable for inclusion in the analysis.7 ,10 ,81-121 ,122 ,123-150 ,151 ,152-179 ,180 ,181-205
OVERALL ANALYSIS
Overall 54 478 patients were studied and a total of 1698 CRCs were detected: 9846 patients had total colitis, among whom 700 cancers were found. Fifty four studies (with 22 730 patients and 844 cancers) included data on age at cancer diagnosis with a mean of 43.2 years (95% CI 40.5–45.9) and 61 studies reported the duration of colitis at cancer diagnosis with a mean of 16.3 years (95% CI 15.0–17.6). There were 75 studies in category 1, 22 in category 2, and 19 in category 3. Table 1 summarises the characteristics of the included trials by category. In the analyses that follow, all studies were not given equal weighting but were weighted proportionally to the number of cases of cancer that were included in the study.
Characteristics of studies included
Considering the overall prevalence of CRC in any patient with UC, based on the total 116 studies, a χ2 test for heterogeneity yielded χ2=799.1, p<0.0001, and therefore a random effects model produced an overall pooled estimate of the prevalence to be 3.7% (95% CI 3.2–4.2). Of the 116 studies, 35 included adequate data on patients with total colitis to calculate the prevalence in this group. In these 35 studies there were 8351 patients with pancolitis and 451 cases of cancer. The χ2=127.5, p<0.0001 and a random effects model produced an overall pooled estimate of the prevalence to be 5.4% (95% CI 4.4–6.5).
ANALYSIS OF STUDIES REPORTING DURATION OF COLITIS (CATEGORIES 2 AND 3)
Of the 116 studies, 41 reported duration of colitis (fig 1). From these studies the overall incidence rate of CRC for any patient with colitis was 3 per 1000 pyd (95% CI 2/1000 to 4/1000). The corresponding annual incidence rate of CRC in the general population given by the Office of National Statistics is 0.6 per 1000 population.206 As 21 of 41 studies did not report the cancer rates at 10 year intervals (and simply gave an overall risk) we had to assume that the cancer risk, in terms of the log incidence rate, remains constant over time. The cumulative probabilities based on this unstratified data gives a risk of 3% (95% CI 2.2–3.8) at 10 years, 5.9% (95% CI 4.3–7.4) at 20 years, and 8.7% (95% CI 6.4–10.9) at 30 years.
Incidence rate of colorectal cancer for any patient with ulcerative colitis, overall and by decade. Ca/pyd, cancer per person years duration.
Twenty six studies in categories 2 and 3 reported data for patients with total colitis and in this group the incidence rate of CRC was 4 per 1000 pyd (95% CI 3/1000 to 6/1000). The unstratified cumulative probabilities gave a risk of 4.4% (95% CI 2.0–6.8) at 10 years, 8.6% (95% CI 4.0–13.3) at 20 years, and 12.7% (95% CI 6.0–19.3) at 30 years.
A further analysis was performed after excluding studies that included referred cancers (two studies) and those that had missing data for this variable (eight studies). This made no statistically significant difference to the results as the overall risk was then 2/1000 pyd (95% CI 2/1000 to 3/1000) and the risk for patients with pancolitis was 4/1000 pyd (95% CI 3/1000 to 5/1000). It was therefore decided to include these 10 studies in further analyses as important information would be lost if they were excluded.
Using overall incidence rates, Egger's test207 was employed to check whether the results could possibly be explained by publication bias. Overall it was found that publication bias was not a statistically significant factor (p=0.46). Egger's test was also used to determine whether language bias could have possibly explained the findings. Studies from countries where English is the first language were compared with those where it is not (and thus one might expect that studies with negative results may have been published in non-English language journals). Again, Egger's test found that language bias was not statistically significant: for studies from the UK, p=0.37; studies from the USA, p=0.47; studies from Scandinavia, p=0.37; and for studies from other countries (namely Iran, Israel, Oman, Czechoslovakia, and Turkey), p=0.90. Furthermore, when English speaking countries were considered collectively, the p value from Egger's test was 0.72 compared with 0.61 from the non-native English speaking countries.
VARIATION OF RISK WITH GEOGRAPHICAL LOCATION
Of the 41 studies, 11 were from the USA,112 ,123 ,129 ,130 ,134 ,144 ,146 ,147 ,153 ,154 ,168 ,18411 from the UK,97 ,98 ,109 ,111 ,118 ,131 ,156 ,160 ,162 ,174 ,190eight from Scandinavia,10 ,119 ,141 ,149 ,152 ,158 ,166 ,183 and 11 were from other countries including Israel, Turkey, Italy, Oman, Iran, Czechoslovakia, and Australia.93 ,100 ,125 ,126 ,139 ,151 ,164 ,169 ,175 ,178 ,179The overall incidence rate for CRC in the USA was 5/1000 pyd (95% CI 3/1000–7/1000), in the UK 4/1000 pyd (95% CI 3/1000–5/1000), in Scandinavia 2/1000 pyd (95% CI 1/1000–3/1000), and in other countries 2/1000 pyd (95% CI 1/1000–4/1000). None of the studies exerted a strong drive towards a particular trend in the meta-analysis.
The geographical incidence rates quoted are based on an overall analysis (of the 41 studies), which therefore assumes that the log incidence rate is constant over time. Because of the smaller numbers of studies that reported results by decade of duration, it was felt that these were insufficient to conduct analyses broken down by country for specific decades.
When the cancer risk for all 41 studies was plotted against the mid point of each study it can be seen that the overall reported cancer incidence has increased from 1955 to the present day (fig 2; size of the circles is proportional to the number of subjects in each study) but the increase is not statistically significant (slope 0.003, p=0.80). The slope can be used to calculate that from studies with a mid point of 1950 to studies with a mid point of 1990 there would be 0.31 extra cases per 1000 pyd. The temporal relationship of CRC risk in each country is also demonstrated. The reported cancer incidence is increasing in all countries but the increase is not statistically significant.
VARIATION OF RISK WITH COLORECTAL SURGERY
The panproctocolectomy rate alone did not exert a statistically significant effect on the CRC risk (z=0.4, p=0.7). When all forms of surgery were considered (panproctocolectomy + resections of varying degree), the reported CRC incidence rateincreased with higher rates of surgical intervention.
ANALYSIS OF STUDIES REPORTING RISK STRATIFIED INTO 10 YEAR INTERVALS (CATEGORY 3)
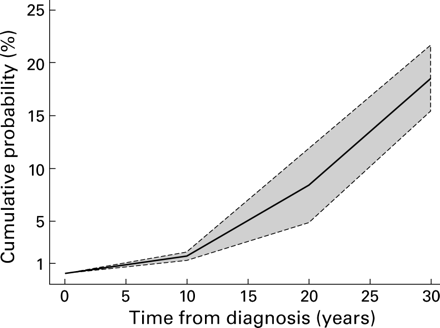
Of the 41 studies, 19 reported results at 10 yearly intervals of disease duration (fig 1). From these studies we were able to estimate how the CRC risk increased with increasing duration of disease and thus stratify the results at 10 year intervals. For the first 10 years duration the overall incidence rate was 2/1000 pyd (95% CI 1/1000–2/1000) while for the second decade of disease the overall incidence rate was estimated to be 7/1000 pyd (95% CI 4/1000–12/1000), and in the third decade of disease the incidence rate was estimated to be 12/1000 pyd (95% CI 7/1000–19/1000). These decade specific incidence rates correspond to a cumulative risk of 1.6% (95% CI 1.2–2) by 10 years, 8.3% (95% CI 4.8–11.7) by 20 years, and 18.4% (95% CI 15.3–21.5) by 30 years (fig 3). The data represented in fig 3 assume that the log incidence rate of CRC is linear over timewithin each 10 year interval, and that changes in the log incidence rate occur at 10, 20, and 30 years. These 10 year intervals correspond with the time points reported in the majority of studies included.
Cumulative risk of developing colorectal cancer for any patient with ulcerative colitis based on stratified data (using stratified incidence, n=19).
Of the 19 studies in category 3, six reported data for patients with total colitis. The stratified decade specific incidence rates for this group were estimated to be 2/1000 pyd (95% CI 1/1000–4/1000) in the first decade, 7/1000 pyd (95% CI 3/1000–14/1000) in the second, and 11/1000 pyd (95% CI 4/1000–28/1000) in the third decade of disease. These decade specific incidence rates correspond to a cumulative risk of 2.1% (95% CI 1.0–3.2%) at 10 years, 8.5% (95% CI 3.8–13.3%) at 20 years, and 17.8% (95% CI 8.3–27.4%) at 30 years.
Table 2 provides a summary of the estimated CRC risks using the separate methods employed.
Summary of estimated cancer risks
To determine if age at onset of UC in adults affected the log incidence rate of CRC, a meta-analysis regression was conducted on 21 studies that reported the age at onset of UC (over 20 years of age). Studies which reported the age at diagnosis of UC were not included as a patient may have had colitis for several years prior to the diagnosis being made. Overall, a negative trend emerged indicating that a younger age at onset in adults was associated with a slightly increased risk of developing cancer, but this was not statistically significant (z=−1.61, p=0.11). A further meta-regression analysis of 11 studies that reported the age at onset of UC together with the risk at 10 yearly intervals also showed that age at onset in adults appeared to have no statistically significant bearing on cancer risk.
ANALYSIS OF STUDIES REPORTING DATA ON CHILDREN ONLY
Eighteen studies in the published literature estimated the incidence of CRC in children with UC. Of these, five were updated by subsequent studies38 ,45-47 ,52 and one included patients with Crohn's disease.83 This left 12 studies suitable for analysis.63 ,66 ,88 ,114 ,134 ,135 ,146 ,147 ,150 ,173 ,180 ,181 ,203Of these only five reported the duration of patient follow up.52 ,63 ,134 ,146 ,147 From these five studies the overall incidence rate of CRC for any child with colitis was 6/1000 pyd (95% CI 3/1000–13/1000). As these studies did not report the numbers of cancers at 10 year intervals, the log incidence rate had to be assumed to be constant. Based on this assumption the cumulative probabilities of any child developing cancer were estimated to be 5.5% (95% CI 2.5–12.3) at 10 years, 10.8% (95% CI 4.8–23.1) at 20 years, and 15.7% (95% CI 7.2–32.6) at 30 years. These rates are higher than the corresponding calculations for adults (3%, 5.9%, and 8.7% respectively). The average age of onset of childhood UC in the five studies was 10 years and the mean duration of follow up was 12 years. Although the other seven studies did not report the mean duration of follow up, the average age of onset of UC was also 10 years and thus it is possible that they too would have given similar rates if they had been included in the analysis.
Discussion
This is the first comprehensive systematic review and meta-analysis assessing the risk of CRC in UC although several reviews have been published addressing this issue.6 ,35 ,76 ,208 ,209 It is also the first meta-analysis of stratified (by duration of disease) cancer incidence rates. The precision of the pooled estimates, both overall and stratified, is due to the relatively large numbers of observations, but the pooled estimates also take into account the between study heterogeneity, as they are based on random effects meta-analysis models. The shortcomings of this meta-analysis are accepted. The methods used have made a number of assumptions and must be applied with caution. Consequently, the results must be interpreted warily. However, in the absence of any large multicentre studies or individual patient data analysis we feel they provide the most accurate method of determining the CRC risk in the current climate.
Most meta-analyses are subject to publication bias as studies with “negative” conclusions are less likely to result in a publication. As there is much debate concerning the risk of CRC in UC, our meta-analysis avoids this bias as authors reporting low rates of CRC in UC are just as likely to have their work published as those reporting very high cancer incidences. This was demonstrated using Egger's test which also showed that language bias does not appear to explain the findings. Although desirable, it was impossible to include unpublished studies in the meta-analysis. There are no registers of observational studies (as there are for clinical trials) and so it is very difficult to identify unpublished data. It could be argued that the analysis was incomplete as only publications in the English language were included. The reason for excluding foreign language studies was the difficulty in extracting information accurately from such studies. If papers in foreign languages had been translated, one would have been dependent on the accuracy of those translations and their not having missed out or misinterpreted data. Furthermore, a Medline search for non-English language articles from the period 1966 to 1999 using CRC, dysplasia, surveillance, and UC as keywords resulted in only three foreign language studies. One of these was in Japanese210 and two were in Portuguese.211 ,212 If this search over the last 33 years is representative of the whole time span since CRC in UC was first reported in 1925, we do not believe a significant number of studies have been missed in the meta-analysis and thus the results are representative.
Other possible biases have been considered. One may argue that the meta-analysis is subject to selection bias in that there may have been a greater chance of inclusion of cases treated by gastroenterologists with the exclusion of cases not treated by gastroenterologists. We feel this to be unlikely as many of the studies in the meta-analysis were population based and their inclusion did not rely on contact with a gastroenterologist. Another possible source of bias is ascertainment bias with a greater likelihood that cancers were detected among those having active follow up. We accept that this may have played a role as the majority of cases came from surveillance programmes or tertiary referral centres and very few studies included in the meta-analysis used national cancer registry data.
From 116 published studies we found that the overall prevalence of CRC in any patient with UC is 3.7% which increases to 5.4% for those with pancolitis. Of the 41 studies that reported duration of disease, the overall incidence of CRC in any patient with UC is estimated at 3/1000 pyd. There is dispute in the literature as to whether young age at onset of colitis is an independent risk factor for CRC. We have found that for any child with UC (irrespective of disease extent) the incidence of CRC is estimated to be 6/1000 pyd which is higher than that calculated for adults. However, this estimate is based on only five studies compared with the 41 in the adult analysis and is thus less accurate, as can be seen by the width of the corresponding confidence intervals.
From the studies that reported the number of cancers at 10 year intervals, we were able to stratify the risk. That is to say we were able to calculate the increase in CRC risk with increasing duration of disease. Such studies led to an estimation of the cumulative risk for any patient with UC to be 2% at 10 years, 8% at 20 years, and 18% at 30 years. Only six studies reported the results by decade for patients with pancolitis and these gave similar cumulative cancer estimates. One expects that the risk of pancolitics would be higher than for any patient with less extensive disease but we feel that the small number of studies in the pancolitis group (n=6) accounts for the similarity in the results, as evidenced by the wide confidence intervals in our calculations. Another factor may have contributed to this apparent similarity in risk. It is unusual that in the articles selected, approximately 20% had total colitis, a value that has been found to pertain to clinical series when it is known that publications must tend towards inclusion of more extensive cases. In this respect, it is noted that only 35 studies in the whole meta-analysis included data on total colitis with other studies not providing sufficient information on disease extent. As over half the cancers developed in patients not stated to have extensive colitis, it suggests that many of these cases did in fact have extensive disease. In this context the similarity of risks between total colitics and all colitics is understandable.
Overall, the incidence of CRC in UC is increasing slightly but this was not statistically significant (fig 2). However, this finding agrees with those of Lashner et al who stated that there was a dramatic increase in the risk of developing CRC in patients with longstanding UC whose disease onset was after 1972 compared with those with disease onset during or before 1972.153 The small increase in the reported cancer incidence may be related to the virtual abolition of performing prophylactic colectomy after approximately 10 years of disease. The period of the meta-analysis also saw the introduction of surveillance strategies for CRC in UC. Although the reported CRC incidence has increased this does not necessarily mean that surveillance has failed. Rather, it is possible that surveillance programmes have led to increased cancer detection.
The incidence of CRC varies with geographical location. In the USA and UK we found that the rate was higher than in Scandinavia and other countries. There are several possible explanations for this finding. It could represent true genetic/environmental population differences relating to the severity or course of the illness, although there is little evidence to suggest that the course of UC varies with country. It is possible that the Western diet plays a role exerting an influence in a similar way to CRC in the general population. Alternatively, it may reflect more active medical therapy strategies to severe disease, particularly in Scandinavia. Aminosalicylates are known to modify disease activity and there is some evidence that they exert some protection against CRC.170 ,213-215 Their protective effect is thought to be mediated in a similar way to aspirin in the general population—that is, by inhibiting mucosal prostaglandin synthesis.216 Indeed, a study from Copenhagen which took an aggressive approach with medical therapy found a very low CRC risk and is the only large published study to report no excess cancer risk.152 Another possible explanation may be variation between countries in the approach to surveillance for CRC. Obviously, centres with a comprehensive programme having high rates of patient follow up (such as St Marks in London) are likely to detect a significantly higher proportion of cancers than centres with less aggressive policies. Finally, studies from hospitals serving a defined catchment area provide a good estimate of cancer risk as it is assumed that cases of all grades of severity are seen. Most of the good data of this type come from Scandinavia119 ,131 and this fact may be part of the reason why the incidence appears lower than in the USA or UK.
It has been suggested that high colectomy rates in patients with UC will reduce the incidence of cancer. This would appear logical as resected bowel obviously no longer has malignant potential. When we analysed this relationship the opposite was found to be true—that is, as more colectomies/resections are performed, cancer incidence is also higher. This is at odds with what one would initially expect but perhaps centres having a low threshold for surgery are also more aggressive in their surveillance strategies and are consequently detecting more cancers by regular colonoscopy. We reviewed the studies with the highest operation rates10 ,112 ,149 as it was not entirely clear whether colectomies had been carried out for cancer prophylaxis or because a cancer had already been identified on barium examination/colonoscopy. However, when these studies were no longer taken into consideration the relationship remained unchanged. It is possible that inclusion of surgical series in this analysis biases the results as some cancers would have been found in operation specimens where the operation was not being carried out for cancer prophylaxis.
In summary, we have estimated as accurately as possible the risk of CRC in UC, having found it to be 2% at 10 years, 8% at 20 years, and 18% at 30 years (irrespective of disease extent). Estimates for patients with total colitis are less reliable due to the smaller number of studies in the analysis. The incidence of CRC in children is higher than that in adults. Incidence rates for CRC are higher in the USA and the UK compared with Scandinavia and other countries. Since 1955 the overall number of cases of CRC in UC has increased but this finding was not statistically significant.
A long term prospective study of colitics with complete follow up would be the most accurate method of assessing the cancer risk in these patients. However, this is an enormous undertaking and is unlikely to be achieved. We have therefore determined the risk from the next best method available to us, a meta-analysis. However, a meta-analysis relies heavily on the quality of data that is reported in published studies. Another option to further refine the estimation of cancer risk would be a meta-analysis of individual patient data from the published literature. However, this assumes that the raw data from studies are still accessible and would require international collaboration between large centres of excellence. The techniques employed here have not been used in this context before and we hope that they may be utilised in future studies to estimate the risk of other diseases in other similar conditions where the incidence is disputed.
Acknowledgments
Part of this meta-analysis was kindly funded by a grant from the Crohn's in Childhood Research Association (CICRA).
STUDY AND PATIENT POPULATION CHARACTERISTICS EXTRACTED
- (1)
- Country of origin.
- (2)
- Type of centre conducting the study and study design.
- (3)
- Period over which the study was conducted.
- (4)
- Number of patients in the study.
- (5)
- Number of patients with total and left sided colitis in the study.
- (6)
- Numbers who developed colorectal cancer (and whether they had total or left sided colitis).
- (7)
- Whether referred cancers were included in the analysis.
- (8)
- The duration of follow up of each study.
- (9)
- The ages of patients at time of onset of UC.
- (10)
- The ages of patients at the time of cancer diagnosis.
- (11)
- The duration of colitis at cancer diagnosis.
- (12)
- The number of patients in the study undergoing panproctocolectomy/partial colectomy.
- (13)
- The cumulative cancer incidence (if reported).
- (14)
- The relative cancer risk (if reported).
- (15)
- The number of patients followed up.
Abbreviations used in this paper
- CRC
- colorectal cancer
- UC
- ulcerative colitis
- pyd
- person years duration