Published online Mar 14, 2010. doi: 10.3748/wjg.v16.i10.1245
Revised: January 20, 2010
Accepted: January 27, 2010
Published online: March 14, 2010
AIM: To identify specific colonoscopic findings in patients with ulcerative colitis (UC) complicated by cytomegalovirus (CMV) infection.
METHODS: Among UC patients who were hospitalized due to exacerbation of symptoms, colonoscopic findings were compared between 15 CMV-positive patients and 58 CMV-negative patients. CMV infection was determined by blood test for CMV antigenemia. Five aspects of mucosal changes were analyzed (loss of vascular pattern, erythema, mucosal edema, easy bleeding, and mucinous exudates) as well as five aspects of ulcerative change (wide mucosal defect, punched-out ulceration, longitudinal ulceration, irregular ulceration, and cobblestone-like appearance). Sensitivity, specificity, positive predictive value, and negative predictive value of each finding for CMV positivity were determined.
RESULTS: The sensitivity of irregular ulceration for positive CMV was 100%. The specificity of wide mucosal defect was 95%. Punched-out ulceration and longitudinal ulceration exhibited relatively high sensitivity and specificity (more than 70% for each).
CONCLUSION: Specific colonoscopic findings in patients with UC complicated by CMV infection were identified. These findings may facilitate the early diagnosis of CMV infection in UC patients.
- Citation: Suzuki H, Kato J, Kuriyama M, Hiraoka S, Kuwaki K, Yamamoto K. Specific endoscopic features of ulcerative colitis complicated by cytomegalovirus infection. World J Gastroenterol 2010; 16(10): 1245-1251
- URL: https://www.wjgnet.com/1007-9327/full/v16/i10/1245.htm
- DOI: https://dx.doi.org/10.3748/wjg.v16.i10.1245
Ulcerative colitis (UC) is a worldwide, chronic, idiopathic, inflammatory disease of the rectal and colonic mucosa. Patients with UC suffer from abdominal pain, diarrhea with blood, tenesmus, and urgency to defecate. In Japan, the number of patients with UC was reported to be only 4400 in 1980. However, the number has increased sharply in recent years, and was estimated to be approximately 100 000 persons in 2008. This number is thought to increase by about 5000 persons each year.
Cytomegalovirus (CMV) belongs to the herpes virus group and is prevalent in adults. Primary CMV infection in immunocompetent people is usually asymptomatic, but the virus remains in the white blood cells throughout the life of the host. Clinically significant diseases occur in patients with suppressed cellular immunity, especially in transplant patients, acquired immunodeficiency syndrome (AIDS) patients, and patients undergoing chemotherapy. The disease is usually the result of reactivation of the latent virus rather than reinfection with the virus. Significant CMV disease may occur in various organs such as the retina, lung, and gastrointestinal tract, and the target organ may be related to the etiology of immunosuppression. In the gastrointestinal tract, CMV disease can occur in all locations from the mouth to the rectum, and usually forms ulcers in the mucosa, often accompanied by hemorrhage[1].
CMV infection has been described as a cause of relapse of inflammatory bowel disease[2-5]. In particular, CMV infection was observed in UC patients, especially those receiving high-dose corticosteroid therapy[4-7]. Moreover, CMV infection can exacerbate the disease[2-5]. Retrospective studies of UC patients have found CMV infection in 5%-21% of surgically resected specimens[2-4,8]. Steroid resistance is also reported to be one of the characteristics of CMV infection in UC[6,8]. In recent prospective studies, 25%-81% of patients with steroid-refractory UC were found to harbor the virus[5-7]. Thus, CMV infection in UC patients can have a severe clinical course and may cause death if appropriate treatment is not given. However, with the development of ganciclovir (GCV) therapy, outcomes have greatly improved[9].
Therefore, it is necessary to make an early diagnosis of CMV infection in UC patients. Although several different methods have been developed to examine CMV infection, such as histology including immunohistochemistry[10,11], serology[10-12], CMV culture[13,14], polymerase chain reaction (PCR) for CMV genome[14-16], and CMV antigenemia[14-16], it is necessary, with regard to clinical aspects, to suspect that UC patients also have CMV infection. In general, the symptoms of UC alone are not sufficient to distinguish exacerbation of UC due to CMV infection from exacerbation of UC unrelated to CMV infection. Specific colonoscopic findings for CMV infection, if any, may facilitate the early diagnosis of CMV infection.
Several reports have examined colonoscopic findings related to CMV infection. However, most of these reports focused on colonoscopic findings in patients with AIDS or in transplant patients[17,18]. The spectrum of colonoscopic findings in those patients was variable and ranged from patchy erythema, exudates, and microerosions to diffusely edematous mucosa, multiple mucosal erosions, deep ulcers and pseudotumors[1,13,17-22]. In contrast, colonoscopic findings of UC complicated by CMV infection have rarely been reported. Only a few studies have described findings without confirmation by statistical analysis[20].
Accordingly, the aim of this study was to identify specific colonoscopic findings in patients with UC complicated by CMV infection. To achieve this, we compared colonoscopic findings in CMV-positive and -negative patients, and determined the sensitivity, specificity, positive predictive value (PPV) and negative predictive value (NPV) of each specific finding for CMV positivity.
This study was a retrospective analysis of medical charts and endoscopic images of UC patients. From January 1999 to August 2007, a total of 200 UC patients were hospitalized in Okayama University Hospital with exacerbation of UC symptoms. We began to routinely examine CMV antigenemia in these patients from 2003. Therefore, of these 200 patients, 111 did not receive a blood test for CMV antigenemia at admission, and 13 did not receive an endoscopic examination. Thus, a total of 76 patients who received both a blood test for CMV antigenemia and an endoscopic examination at admission were analyzed in this study. This study was approved by the institutional review board of Okayama University Graduate School of Medicine, Dentistry, and Pharmaceutical Sciences.
The diseases in these patients were pancolitis or left-sided colitis with moderate or severe activity, based on clinical, endoscopic, and histological findings according to established criteria[23-25]. Medical charts provided clinical parameters including demographic data such as age, gender, and duration of the disease, as well as disease status such as severity of disease, extent of disease, and medications.
Diagnosis of CMV infection was determined by CMV antigenemia, which was examined by detecting CMV pp65 antigen in peripheral blood leukocytes using the direct immunoperoxidase technique with horseradish peroxidase-conjugated Fab’ fragment of human monoclonal antibody (C7-HRP) and immunofluorescence staining with monoclonal antibodies C10/11. The examinations were performed in the laboratory of SRL Inc. (Tokyo, Japan). We defined a patient as CMV-positive when one or more positive cells of either C7-HRP or C10/11 were detected in more than 10 000 leukocytes (the minimum number of positive cells).
Endoscopic findings were evaluated by examining recorded colonoscopic images and reports. Two aspects of colonic changes were assessed: mucosal change and ulcerative change. Mucosal change was evaluated according to the following five features: easy bleeding, loss of vascular pattern, mucosal edema, erythema, and mucinous exudates. Each item was expressed as “severe” or “not severe” according to the severity of each change. Easy bleeding was defined as bleeding from the fragile membrane due to slight contact of the endoscope. Loss of vascular pattern was loss of visible vessels under the membrane, generally seen during colonoscopy. Mucosal edema was defined as thick and swollen membrane which pooled fluid. Erythema was red coloration of the membrane composed of dilated veins. Mucinous exudates were exudates composed of blood and pus.
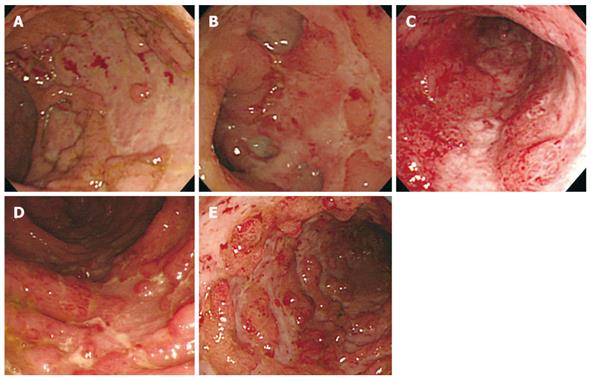
For ulcerative changes, we determined whether or not the following forms of ulceration were observed: wide mucosal defect, punched-out ulceration, longitudinal ulceration, irregular ulceration, and cobblestone-like appearance. A wide mucosal defect was a wide area of this defect with a longitudinal and/or transverse spread, indicating that more than one-fourth of the mucosa in the endoscopic field was defective (Figure 1A). Punched-out ulceration formed an almost round shape of ulceration with clear demarcation (Figure 1B). Longitudinal ulceration had a longitudinal spread along the lumen of the colon (Figure 1C). Irregular ulceration was defined as ulceration with an irregular pattern and a branched shape (Figure 1D). Cobblestone-like appearance was an aggregate of elevated lesions as a result of the changes in the membrane, which looked like half-spheres of subpedunculated polyps with multiple ulceration as seen in Crohn’s disease (Figure 1E). Each endoscopic image was evaluated by two (Suzuki H and Kato J) of the authors. If there were any disagreements in these evaluations, a third reviewer (Hiraoka S) made the final evaluation. At the time of interpretation of the images, the CMV antigenemia results were blinded to interpreters. The rate of disagreements between the two authors was less than 5%.
Of the clinical characteristics, the χ2 test and Fisher’s exact test were used to compare two discrete variables. The significance of differences for continuous variables was assessed by the Mann-Whitney U-test. Of the endoscopic findings, we calculated sensitivity, specificity, PPV, and NPV for CMV positivity. In addition, odds ratios (OR) with 95% CI were determined by a univariate logistic regression model. The results were considered statistically significant when P values were less than 0.05. Statistical analysis of the data was carried out with SAS software version 9.2 (SAS Institute, Cary, NC, USA).
A total of 76 patients received a blood test for CMV antigenemia and a colonoscopy at admission. Of these, 18 patients were positive for CMV antigenemia, while the remaining 58 patients were negative. Of the 18 CMV-positive patients, symptoms in 3 patients were relatively mild, and those patients entered remission without antiviral therapy. The remaining 15 patients required GCV therapy for a period of two weeks, together with dose reduction of corticosteroids. As a consequence of these treatments, all patients entered remission. Therefore, a comparative analysis was performed on 15 CMV-positive patients vs 58 CMV-negative patients.
The clinical characteristics of these 73 patients are shown in Table 1. There were no significant differences in clinical characteristics such as gender, age, duration of disease, disease activity, extent of disease, use of corticosteroids, and use of immunomodulators (azathioprine or mercaptopurine) between CMV-positive and -negative patients. It is noteworthy that all the CMV-positive patients received corticosteroid therapy.
| CMV positive (n = 15) | CMV negative (n = 58) | P value | |
| Gender | |||
| Male | 8 (53) | 26 (45) | 0.55 |
| Female | 7 (47) | 32 (55) | |
| Age (yr), median (range) | 42 (17-65) | 34 (14-81) | 0.72 |
| Duration of disease (d), median (range) | 528 (15-9358) | 1503 (5-10 791) | 0.40 |
| Disease activity at admission | |||
| Moderate | 9 (60) | 41 (71) | 0.43 |
| Severe | 6 (40) | 17 (29) | |
| Extent of disease | |||
| Pancolitis | 12 (80) | 42 (72) | 0.74 |
| Left-sided | 3 (20) | 16 (28) | |
| Current use of corticosteroids | |||
| Yes | 15 (100) | 47 (81) | 0.11 |
| No | 0 (0) | 11 (19) | |
| Current use of immunomodulators (AZA/6-MP) | |||
| Yes | 1 (7) | 7 (12) | > 0.99 |
| No | 14 (93) | 51 (88) |
To identify specific endoscopic findings of UC complicated by CMV infection, five aspects of mucosal changes (loss of vascular pattern, erythema, mucosal edema, easy bleeding, and mucinous exudates) as well as five aspects of ulcerative change (wide mucosal defect, punched-out ulceration, longitudinal ulceration, irregular ulceration, and cobblestone-like appearance) were analyzed. The sensitivity, specificity, PPV and NPV of each finding for CMV positivity were determined. In addition, univariate logistic regression analyses were performed to determine the OR and 95% CI of each finding (Table 2).
| CMV positive (n = 15) | CMV negative (n = 58) | Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) | Odds ratio | 95% CI | P value | |
| Easy bleeding | |||||||||
| Severe | 6 (40) | 7 (12) | 40 | 88 | 46 | 85 | 2.20 | 1.14-4.28 | 0.02 |
| Not severe | 9 (60) | 51 (88) | |||||||
| Loss of the vascular pattern | |||||||||
| Severe | 13 (87) | 37 (64) | 87 | 36 | 26 | 91 | 1.92 | 0.95-5.01 | 0.11 |
| Not severe | 2 (13) | 21 (36) | |||||||
| Edema | |||||||||
| Severe | 12 (80) | 33 (57) | 80 | 43 | 27 | 89 | 1.74 | 0.92-3.79 | 0.11 |
| Not severe | 3 (20) | 25 (43) | |||||||
| Erythema | |||||||||
| Severe | 13 (87) | 39 (67) | 87 | 33 | 25 | 90 | 1.78 | 0.88-4.64 | 0.15 |
| Not severe | 2 (13) | 19 (33) | |||||||
| Mucinous exudates | |||||||||
| Severe | 4 (27) | 11 (19) | 27 | 81 | 27 | 81 | 1.25 | 0.61-2.36 | 0.51 |
| Not severe | 11 (73) | 47 (81) | |||||||
| Wide mucosal defect | |||||||||
| ≥ 1/4 field | 8 (53) | 3 (5) | 53 | 95 | 72 | 89 | 4.58 | 2.21-10.73 | 0.0001 |
| < 1/4 field | 7 (47) | 55 (95) | |||||||
| Punched-out ulceration | |||||||||
| Yes | 12 (80) | 15 (26) | 80 | 74 | 44 | 93 | 3.39 | 1.78-7.46 | 0.0006 |
| No | 3 (20) | 43 (74) | |||||||
| Longitudinal ulceration | |||||||||
| Yes | 11 (73) | 13 (22) | 73 | 78 | 46 | 92 | 3.09 | 1.66-6.26 | 0.0007 |
| No | 4 (27) | 45 (78) | |||||||
| Irregular ulceration | |||||||||
| Yes | 15 (100) | 34 (59) | 100 | 41 | 31 | 100 | ND | ND | ND |
| No | 0 (0) | 24 (41) | |||||||
| Cobblestone-like appearance | |||||||||
| Yes | 7 (47) | 10 (17) | 47 | 83 | 41 | 86 | 2.05 | 1.11-3.82 | 0.02 |
| No | 8 (53) | 48 (83) |
Of the 5 mucosal changes, loss of vascular pattern, edema, and erythema exhibited relatively high sensitivity for CMV positivity (87%, 80%, and 87%, respectively), whereas easy bleeding and mucinous exudates exhibited high specificity (88% and 81%, respectively). Of the 5 ulcerative changes, irregular ulceration showed 100% sensitivity, but relatively low specificity (41%). Wide mucosal defect and cobblestone-like appearance showed relatively high specificity (95% and 83%, respectively), but low sensitivity. The sensitivity and specificity of punched-out ulceration and longitudinal ulceration were both relatively high (punched-out ulceration, sensitivity 80%, specificity 74%, longitudinal ulceration sensitivity 73%, specificity 78%, respectively).
Univariate logistic regression analysis revealed that severe easy bleeding was seen more frequently in CMV-positive patients than in CMV-negative patients (OR = 2.20, 95% CI: 1.14-4.28). Wide mucosal defect (OR = 4.58, 95% CI: 2.21-10.73), punched-out ulceration (OR = 3.39, 95% CI: 1.78-7.46), longitudinal ulceration (OR = 3.09, 95% CI: 1.66-6.26), and cobblestone-like appearance (OR = 2.05, 95% CI: 1.11-3.82) were more frequently observed in CMV-positive patients than in CMV-negative patients.
Colonoscopy is generally performed in UC patients who undergo flare-ups. Direct observation of the colon provides detailed information on the disease status and is useful for comprehending the severity of disease, and thus it is an important tool for formulating treatments. However, although CMV infection can cause poor prognosis in UC patients (i.e. a high chance of colectomy), reports on the specific colonoscopic findings of UC with CMV infection are scarce. Therefore, in this study we analyzed the colonoscopic findings of UC patients with and without CMV infection, and showed that there were specific findings that predicted CMV infection in UC patients.
There have been reports on the endoscopic findings of CMV colitis in AIDS or transplant patients. These findings include patchy erythema, exudates, microerosions, diffusely edematous mucosa, multiple mucosal erosions, or deep ulcers and pseudotumors[1,13,17-22]. In UC patients, however, the findings would be more complicated, because inflammatory changes due to UC itself in colonic mucosa exists prior to CMV infection. Therefore, in previous studies, no specific endoscopic findings were observed in UC patients with concomitant CMV infection[6,26]. In contrast, in our study, specific colonoscopic findings of UC with CMV infection were observed; the sensitivity of irregular ulceration was 100%, the specificity of wide mucosal defect was 95%, and the sensitivity and specificity of punched-out ulceration and longitudinal ulceration were both greater than 70%. Therefore, in cases where these colonoscopic findings are seen in UC patients, examination to confirm CMV infection should be performed as soon as possible.
It is known that UC patients with CMV infection, especially those who are compromised by corticosteroid therapy, experience severe symptoms. These patients are likely to undergo colectomy, and CMV infection has been confirmed in 4.6%-25% of patients who have undergone colectomy[2,3,7,8]. Recently the efficacy of GCV in CMV infection has become widely recognized. Pfau et al[9] considers GCV a beneficial treatment that significantly decreases the mortality rate and the need for surgery. Wada et al[6] found that GCV was effective in 8 (67%) of 12 patients with CMV infection. They also reported that steroid withdrawal improved steroid-resistant UC. Matsuoka et al[27] reported that most CMV reactivation-positive patients responded to conventional immunosuppressive therapies, however, high values of CMV antigenemia may be an indicator for antiviral treatment regardless of histology. Thus, treatment for UC patients with CMV infection should be different from that for UC patients without CMV infection. Therefore, our findings that predict CMV infection should be useful in therapeutic decision-making for UC patients.
In addition, there are several studies on the prophylactic/pre-emptive therapy of CMV diseases in patients with solid organ transplants, hematopoietic stem cell transplants, and human immunodeficiency virus disease[27-29]. These reports suggested the importance of early detection of CMV reactivation and early administration of anti-viral drugs before the development of CMV disease. Although no concept of prophylactic therapy has been established for UC patients, early detection and early antiviral therapy may be considered because of the high rate of colectomy in these patients. In this context, early prediction of CMV infection in UC patients by colonoscopy would seem helpful in clinical settings.
There are several methods of detecting CMV infection: histology including immunohistochemistry[10,11], serology[10-12], CMV culture[13,14], PCR for CMV genome[14-16], and CMV antigenemia[14-16]. Each method has advantages and disadvantages for the precise diagnosis of CMV infection. For example, histological examination is a relatively easy method, but the sensitivity is not so high (10%-87%)[10-12]. Meanwhile, PCR for the CMV genome is very sensitive, but the false-positive rate is relatively high. In our analysis, CMV antigenemia was adopted to detect CMV infection, because examination of CMV antigenemia is relatively sensitive (60%-100%) and easy to measure within a short period[10,14,16,30]. In addition, this method has been used for monitoring CMV infection in heart transplant recipients, and for the early diagnosis of CMV infection in renal transplant recipients[29]. Moreover, it has been reported that positive or negative results of CMV antigenemia are a good indication for antiviral therapy[28,31]. In fact, in our study, 15 (83%) of 18 patients who were positive for CMV antigenemia required antiviral therapy with GCV, and all of these patients entered remission without colectomy. In general, immunohistochemistry is considered the gold standard in the diagnosis of CMV infection. In our cohort, however, among 6 patients with positive CMV antigenemia whose biopsy specimens were immunostained with antibody for CMV, only 3 exhibited positive immunostaining. In addition, although we performed immunostaining in 16 of 58 patients with negative CMV antigenemia, none of them exhibited positive staining. Therefore, the use of CMV antigenemia for CMV detection in this study was appropriate.
Our study has several limitations. First, CMV infection was determined by CMV antigenemia alone. As described above, there are several methods to detect CMV infection. A combination of these methods may have increased the CMV detection rate. Second, of the 18 CMV antigenemia-positive patients, 15 patients who were given GCV were analyzed in our study. However, it is not certain that all 15 patients really required GCV therapy. Some of these patients may have improved without GCV similar to the remaining 3 patients. Third, this is a retrospective study that relied on the examination of recorded colonoscopic images. Therefore, bias due to patient collection and missed recording of colonoscopic findings may inevitably exist. Finally, this is a relatively small scale study in Japan, and it is possible that the results might differ in patients of other ethnicities from other parts of the world.
In the case of a severe flare-up of UC, the possibility of a concurrent CMV infection causing or worsening colitis should be considered, especially when patients are on immunosuppressive medications that may make them more susceptible to this viral infection. The prognosis of UC cases with CMV infection is considered poor. Thus, CMV infection should be closely investigated in such cases, and anti-CMV treatment (GCV) should be given immediately to reduce the rate of colectomy. The specific colonoscopic findings of patients with UC complicated by CMV infection observed in this study may facilitate early diagnosis and treatment of CMV infection.
In general, the symptoms of ulcerative colitis (UC) alone are not sufficient to distinguish exacerbation of UC due to CMV infection from exacerbation of UC unrelated to cytomegalovirus (CMV) infection.
Few studies have reported the colonoscopic findings of UC complicated by CMV infection.
Via retrospective research, authors found specific colonoscopic findings in UC patients with CMV antigenemia.
Specific colonoscopic findings in patients with UC complicated by CMV infection found in this study may facilitate early diagnosis and treatment of CMV infection.
This is a potentially important study that is generally well done, well written, and makes a contribution to the field.
Peer reviewers: Marc Basson, MD, PhD, MBA, Chief of Surgery, John D. Dingell VA Medical Center, 4646 John R. Street, Detroit, MI 48301, United States; Sri P Misra, Professor, Gastroenterology, Moti Lal Nehru Medical College, Allahabad 211001, India
S- Editor Tian L L- Editor Webster JR E- Editor Ma WH
| 1. | Goodgame RW. Gastrointestinal cytomegalovirus disease. Ann Intern Med. 1993;119:924-935. [Cited in This Article: ] |
| 2. | Kaufman HS, Kahn AC, Iacobuzio-Donahue C, Talamini MA, Lillemoe KD, Hamilton SR. Cytomegaloviral enterocolitis: clinical associations and outcome. Dis Colon Rectum. 1999;42:24-30. [Cited in This Article: ] |
| 3. | Takahashi Y, Tange T. Prevalence of cytomegalovirus infection in inflammatory bowel disease patients. Dis Colon Rectum. 2004;47:722-726. [Cited in This Article: ] |
| 4. | Berk T, Gordon SJ, Choi HY, Cooper HS. Cytomegalovirus infection of the colon: a possible role in exacerbations of inflammatory bowel disease. Am J Gastroenterol. 1985;80:355-360. [Cited in This Article: ] |
| 5. | Cottone M, Pietrosi G, Martorana G, Casà A, Pecoraro G, Oliva L, Orlando A, Rosselli M, Rizzo A, Pagliaro L. Prevalence of cytomegalovirus infection in severe refractory ulcerative and Crohn's colitis. Am J Gastroenterol. 2001;96:773-775. [Cited in This Article: ] |
| 6. | Wada Y, Matsui T, Matake H, Sakurai T, Yamamoto J, Kikuchi Y, Yorioka M, Tsuda S, Yao T, Yao S. Intractable ulcerative colitis caused by cytomegalovirus infection: a prospective study on prevalence, diagnosis, and treatment. Dis Colon Rectum. 2003;46:S59-S65. [Cited in This Article: ] |
| 7. | Kambham N, Vij R, Cartwright CA, Longacre T. Cytomegalovirus infection in steroid-refractory ulcerative colitis: a case-control study. Am J Surg Pathol. 2004;28:365-373. [Cited in This Article: ] |
| 8. | Cooper HS, Raffensperger EC, Jonas L, Fitts WT Jr. Cytomegalovirus inclusions in patients with ulcerative colitis and toxic dilation requiring colonic resection. Gastroenterology. 1977;72:1253-1256. [Cited in This Article: ] |
| 9. | Pfau P, Kochman ML, Furth EE, Lichtenstein GR. Cytomegalovirus colitis complicating ulcerative colitis in the steroid-naive patient. Am J Gastroenterol. 2001;96:895-899. [Cited in This Article: ] |
| 10. | de la Hoz RE, Stephens G, Sherlock C. Diagnosis and treatment approaches of CMV infections in adult patients. J Clin Virol. 2002;25 Suppl 2:S1-S12. [Cited in This Article: ] |
| 11. | Beaugerie L, Cywiner-Golenzer C, Monfort L, Girard PM, Carbonnel F, Ngô Y, Cosnes J, Rozenbaum W, Nicolas JC, Châtelet FP. Definition and diagnosis of cytomegalovirus colitis in patients infected by human immunodeficiency virus. J Acquir Immune Defic Syndr Hum Retrovirol. 1997;14:423-429. [Cited in This Article: ] |
| 12. | Kishore J, Ghoshal U, Ghoshal UC, Krishnani N, Kumar S, Singh M, Ayyagari A. Infection with cytomegalovirus in patients with inflammatory bowel disease: prevalence, clinical significance and outcome. J Med Microbiol. 2004;53:1155-1160. [Cited in This Article: ] |
| 13. | Meyers JD, Ljungman P, Fisher LD. Cytomegalovirus excretion as a predictor of cytomegalovirus disease after marrow transplantation: importance of cytomegalovirus viremia. J Infect Dis. 1990;162:373-380. [Cited in This Article: ] |
| 14. | Boivin G, Handfield J, Toma E, Murray G, Lalonde R, Tevere VJ, Sun R, Bergeron MG. Evaluation of the AMPLICOR cytomegalovirus test with specimens from human immunodeficiency virus-infected subjects. J Clin Microbiol. 1998;36:2509-2513. [Cited in This Article: ] |
| 15. | Mazzulli T, Drew LW, Yen-Lieberman B, Jekic-McMullen D, Kohn DJ, Isada C, Moussa G, Chua R, Walmsley S. Multicenter comparison of the digene hybrid capture CMV DNA assay (version 2.0), the pp65 antigenemia assay, and cell culture for detection of cytomegalovirus viremia. J Clin Microbiol. 1999;37:958-963. [Cited in This Article: ] |
| 16. | Rowshani AT, Bemelman FJ, van Leeuwen EM, van Lier RA, ten Berge IJ. Clinical and immunologic aspects of cytomegalovirus infection in solid organ transplant recipients. Transplantation. 2005;79:381-386. [Cited in This Article: ] |
| 17. | Wilcox CM, Chalasani N, Lazenby A, Schwartz DA. Cytomegalovirus colitis in acquired immunodeficiency syndrome: a clinical and endoscopic study. Gastrointest Endosc. 1998;48:39-43. [Cited in This Article: ] |
| 18. | Battaglino MP, Rockey DC. Cytomegalovirus colitis presenting with the endoscopic appearance of pseudomembranous colitis. Gastrointest Endosc. 1999;50:697-700. [Cited in This Article: ] |
| 19. | Ljungman P, Griffiths P, Paya C. Definitions of cytomegalovirus infection and disease in transplant recipients. Clin Infect Dis. 2002;34:1094-1097. [Cited in This Article: ] |
| 20. | Nishimoto Y, Matsumoto T, Suekane H, Shimizu M, Mikami Y, Iida M. Cytomegalovirus infection in a patient with ulcerative colitis: colonoscopic findings. Gastrointest Endosc. 2001;53:816-818. [Cited in This Article: ] |
| 21. | Falagas ME, Griffiths J, Prekezes J, Worthington M. Cytomegalovirus colitis mimicking colon carcinoma in an HIV-negative patient with chronic renal failure. Am J Gastroenterol. 1996;91:168-169. [Cited in This Article: ] |
| 22. | Roskell DE, Hyde GM, Campbell AP, Jewell DP, Gray W. HIV associated cytomegalovirus colitis as a mimic of inflammatory bowel disease. Gut. 1995;37:148-150. [Cited in This Article: ] |
| 23. | Lennard-Jones JE. Classification of inflammatory bowel disease. Scand J Gastroenterol Suppl. 1989;170:2-6; discussion 16-19. [Cited in This Article: ] |
| 24. | Gower-Rousseau C, Salomez JL, Dupas JL, Marti R, Nuttens MC, Votte A, Lemahieu M, Lemaire B, Colombel JF, Cortot A. Incidence of inflammatory bowel disease in northern France (1988-1990). Gut. 1994;35:1433-1438. [Cited in This Article: ] |
| 25. | Truelove SC, Witts LJ. Cortisone in ulcerative colitis; final report on a therapeutic trial. Br Med J. 1955;2:1041-1048. [Cited in This Article: ] |
| 26. | Sakamoto I, Shirai T, Kamide T, Igarashi M, Koike J, Ito A, Takagi A, Miwa T, Kajiwara H. Cytomegalovirus enterocolitis in an immunocompetent individual. J Clin Gastroenterol. 2002;34:243-246. [Cited in This Article: ] |
| 27. | Matsuoka K, Iwao Y, Mori T, Sakuraba A, Yajima T, Hisamatsu T, Okamoto S, Morohoshi Y, Izumiya M, Ichikawa H. Cytomegalovirus is frequently reactivated and disappears without antiviral agents in ulcerative colitis patients. Am J Gastroenterol. 2007;102:331-337. [Cited in This Article: ] |
| 28. | Manteiga R, Martino R, Sureda A, Labeaga R, Brunet S, Sierra J, Rabella N. Cytomegalovirus pp65 antigenemia-guided pre-emptive treatment with ganciclovir after allogeneic stem transplantation: a single-center experience. Bone Marrow. Transplant. 1998;22:899-904. [Cited in This Article: ] |
| 29. | Bernabeu-Wittel M, Pachón-Ibáñez J, Cisneros JM, Cañas E, Sánchez M, Gómez MA, Gentil MA, Pachón J. Quantitative pp65 antigenemia in the diagnosis of cytomegalovirus disease: prospective assessment in a cohort of solid organ transplant recipients. J Infect. 2005;51:188-194. [Cited in This Article: ] |
| 30. | Mori T, Okamoto S, Matsuoka S, Yajima T, Wakui M, Watanabe R, Ishida A, Iwao Y, Mukai M, Hibi T. Risk-adapted pre-emptive therapy for cytomegalovirus disease in patients undergoing allogeneic bone marrow transplantation. Bone Marrow Transplant. 2000;25:765-769. [Cited in This Article: ] |
| 31. | Boeckh M, Gooley TA, Myerson D, Cunningham T, Schoch G, Bowden RA. Cytomegalovirus pp65 antigenemia-guided early treatment with ganciclovir versus ganciclovir at engraftment after allogeneic marrow transplantation: a randomized double-blind study. Blood. 1996;88:4063-4071. [Cited in This Article: ] |