Abstract
Background and Objective
Trans-resveratrol is a polyphenol, which is found in red wine and has cancer chemo-preventive properties and disease-preventive properties. The pharmacokinetics of trans-resveratrol have been investigated in single-dose studies and in studies with relatively low dosages. The present study aimed to investigate the steady-state pharmacokinetics and tolerability of trans-resveratrol 2000 mg twice daily with food, quercetin and alcohol (ethanol).
Methods
This was a two-period, open-label, single-arm, within-subject control study in eight healthy subjects. The steady-state 12-hour pharmacokinetics of trans-resveratrol 2000 mg twice daily were studied with a standard breakfast, a high-fat breakfast, quercetin 500 mg twice daily and 5% alcohol 100 mL. Trans-resveratrol plasma concentrations were determined using liquid chromatography with tandem mass spectrometry.
Results
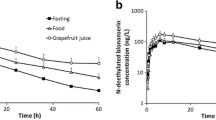
The mean (SD) area under the plasma concentration-time curve from 0 to 12 hours (AUC12) and maximum plasma concentration (Cmax) of trans-resveratrol were 3558 (2195) ng •h/mL and 1274 (790) ng/mL, respectively, after the standard breakfast. The high-fat breakfast significantly decreased the AUC12 and Cmax by 45% and 46%, respectively, when compared with the standard breakfast. Quercetin 500 mg twice daily or 5% alcohol 100mL did not influence trans-resveratrol pharmacokinetics. Diarrhoea was reported in six of the eight subjects. Significant but not clinically relevant changes from baseline were observed in serum potassium and total bilirubin levels.
Conclusion
Trans-resveratrol 2000 mg twice daily resulted in adequate exposure and was well tolerated by healthy subjects, although diarrhoea was frequently observed. In order to maximize trans-resveratrol exposure, it should be taken with a standard breakfast and not with a high-fat meal. Furthermore, combined intake with quercetin or alcohol did not influence trans-resveratrol exposure.




Similar content being viewed by others
References
Granados-Soto V. Pleiotropic effects of resveratrol. Drug News Perspect 2003 Jun; 16(5): 299–307
Guarente L, Picard F. Calorie restriction: the SIR2 connection. Cell 2005 Feb 25; 120(4): 473–82
Soleas GJ, Grass L, Josephy PD, et al. A comparison of the anticarcinogenic propertiesoffour red wine polyphenols. Clin Biochem 2006 May; 39(5): 492–7
Whitsett Jr TG, Lamartiniere CA. Genistein and resveratrol: mammary cancer chemoprevention and mechanisms of action in the rat. Expert Rev Anticancer Ther 2006 Dec; 6(12): 1699–706
Ramassamy C. Emerging role of polyphenolic compounds in the treatment of neurodegenerative diseases: a review of their intracellular targets. Eur J Pharmacol 2006 Sep 1; 545(1): 51–64
Bujanda L, Garcia-Barcina M, Gutierrez-de Juan V, et al. Effect of resveratrol on alcohol-induced mortality and liver lesions in mice. BMC Gastroenterol 2006; 6: 35
Wang Q, Xu J, Rottinghaus GE, et al. Resveratrol protects against global cerebral ischemic injury in gerbils. Brain Res 2002 Dec 27; 958(2): 439–47
Pace-Asciak CR, Hahn S, Diamandis EP, et al. The red wine phenolics trans-resveratrol and quercetin block human platelet aggregation and eicosanoid synthesis: implications for protection against coronary heart disease. Clin Chim Acta 1995 Mar 31; 235(2): 207–19
Walle T, Hsieh F, DeLegge MH, et al. High absorption but very low bioavailability of oral resveratrol in humans. Drug Metab Dispos 2004 Dec; 32(12): 1377–82
Boocock DJ, Faust GE, Patel KR, et al. Phase I dose escalation pharm-acokinetic studyin healthy volunteersof resveratrol, apotential cancer chemo-preventive agent. Cancer Epidemiol Biomarkers Prev 2007 Jun; 16(6): 1246–52
Almeida L, Vaz-da-Silva M, Falcao A, et al. Pharmacokinetic and safety profile of trans-resveratrol in a rising multiple-dose study in healthy volunteers. Mol Nutr Food Res 2009 May; 53(S1): S7–15
Vaz-da-Silva M, Loureiro AI, Falcao A, et al. Effect of food on the pharm-acokinetic profile of trans-resveratrol. Int J Clin Pharmacol Ther 2008 Nov; 46(11): 564–70
De Santi C, Pietrabissa A, Spisni R, et al. Sulphation of resveratrol, a natural compound present in wine, and its inhibition by natural flavonoids. Xeno-biotica 2000 Sep; 30(9): 857–66
Goldberg DM, Yan J, Soleas GJ. Absorption of three wine-related polyphenols in three different matrices by healthy subjects. Clin Biochem 2003 Feb; 36(1): 79–87
Gescher AJ, Steward WP. Relationship between mechanisms, bioavailability, and preclinical chemopreventive efficacy ofresveratrol: a conundrum. Cancer Epidemiol Biomarkers Prev 2003 Oct; 12(10): 953–7
Acknowledgements
The healthy subjects are gratefully acknowledged for their participation in this study. D. William Cameron is supported by a Career Scientist Award from the Ontario Ministry of Health/Ontario HIV Treatment Network. No sources of funding were used for the conduct of this study or preparation of this manuscript. The authors have no conflicts of interest that are directly relevant to the content of this study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
la Porte, C., Voduc, N., Zhang, G. et al. Steady-State Pharmacokinetics and Tolerability of Trans-Resveratrol 2000mg Twice Daily with Food, Quercetin and Alcohol (Ethanol) in Healthy Human Subjects. Clin Pharmacokinet 49, 449–454 (2010). https://doi.org/10.2165/11531820-000000000-00000
Published:
Issue Date:
DOI: https://doi.org/10.2165/11531820-000000000-00000