-
PDF
- Split View
-
Views
-
Cite
Cite
Jessica B. O’Connell, Melinda A. Maggard, Clifford Y. Ko, Colon Cancer Survival Rates With the New American Joint Committee on Cancer Sixth Edition Staging, JNCI: Journal of the National Cancer Institute, Volume 96, Issue 19, 6 October 2004, Pages 1420–1425, https://doi.org/10.1093/jnci/djh275
Close - Share Icon Share
Abstract
Background: The recently revised American Joint Committee on Cancer (AJCC) sixth edition cancer staging system increased the stratification within colon cancer stages II and III defined by the AJCC fifth edition system. Using nationally representative Surveillance, Epidemiology, and End Results (SEER) data, we compared survival rates associated with colon cancer stages defined according to both AJCC systems. Methods: Using SEER data (from January 1, 1991, through December 31, 2000), we identified 119 363 patients with colon adenocarcinoma and included all patients in two analyses by stages defined by AJCC fifth and sixth edition systems. Tumors were stratified by SEER’s “extent of disease” and “number of positive [lymph] nodes” coding schemes. Kaplan–Meier analyses were used to compare overall and stage-specific 5-year survival. All statistical tests were two-sided. Results: Overall 5-year survival was 65.2%. According to stages defined by the AJCC fifth edition system, 5-year stage-specific survivals were 93.2% for stage I, 82.5% for stage II, 59.5% for stage III, and 8.1% for stage IV. According to stages defined by the AJCC sixth edition system, 5-year stage-specific survivals were 93.2% for stage I, 84.7% for stage IIa, 72.2% for stage IIb, 83.4% for stage IIIa, 64.1% for stage IIIb, 44.3% for stage IIIc, and 8.1% for stage IV. Under the sixth edition system, 5-year survival was statistically significantly better for patients with stage IIIa colon cancer (83.4%) than for patients with stage IIb disease (72.2%) (P<.001). Conclusions: The AJCC sixth edition system for colon cancer stratifies survival more distinctly than the fifth edition system by providing more substages. The association of stage IIIa colon cancer with statistically significantly better survival than stage IIb in the new system may reflect current clinical practice, in which stage III patients receive chemotherapy but stage II patients generally do not.
Since 1959, the American Joint Committee on Cancer (AJCC) has been working to formulate systems of classification for cancer staging. These systems are designed to enable physicians to stratify patients in terms of expected predicted survival, to help select the most effective treatments, to determine prognoses, and to evaluate cancer control measures (1,2).
As of January 1, 2003, the newest edition of the AJCC Cancer Staging Manual, sixth edition, is being used to stage all new cancer cases. This edition contains updated classification schemes for several cancers, including colon cancer. The prior (fifth edition) staging system for colon cancer had four categories that were based on the tumor–node–metastasis (TNM) classification (stages I, II, III, and IV). The new (sixth edition) staging system for colon cancer stratifies stages II and III further by use of the T stage (i.e., tumor depth of penetration) and the N stage (i.e., number of positive lymph nodes), resulting in a total of seven stages (stages I, IIa, IIb, IIIa, IIIb, IIIc, and IV; Table 1) (1).
In this article, we use data from the Surveillance, Epidemiology, and End Results (SEER1) program, a large national cancer registry, to compare survival outcomes by stage as defined by both the older (fifth edition) and the newer (sixth edition) AJCC staging systems for colon cancer. We also evaluate other factors, in addition to TNM stages, as potential prognostic factors.
Patients and Methods
We identified and evaluated all 119 363 patients diagnosed with colon adenocarcinoma in the SEER national cancer registry from January 1, 1991, through December 31, 2000. The SEER program collects patient records from multiple sites across the United States and is regarded as a model population-based tumor registry. This national program includes 12 regional registries that cover approximately 14% of the U.S. population. The database was designed to reflect the overall characteristics of the U.S. population, including the diverse array of racial and/or ethnic groups, geographic locations, and types of cities and states (3).
We characterized tumors according to the location in the colon: cecum, ascending colon, hepatic flexure, transverse colon, splenic flexure, descending colon, sigmoid colon, and large intestine NOS (not otherwise specified). Appendiceal, rectosigmoid, and rectal locations were excluded, as were in situ tumors. We further restricted the tumors included by specific histologic type, as defined by the following individual International Classification of Diseases for Oncology, second edition, codes for adenocarcinomas (8010, 8020–8022, 8140–8145, 8210–8211, 8220–8221, 8230–8231, 8260–8263), mucinous adenocarcinomas (8470, 8480, 8481), and signet ring cell carcinoma (9490).
Patient Demographics
Demographic information recorded for each patient included age, sex, race, and/or ethnicity (white, black, Hispanic, Asian, or “other”), marital status (single or married at diagnosis), and SEER registry site (Alaska, Atlanta, Connecticut, Detroit, Hawaii, Iowa, Los Angeles [after 1992], New Mexico, San Francisco, San Jose [after 1992], Seattle, and Utah).
Tumor and Disease Characteristics
Cancer-specific data evaluated for each patient included stage at presentation, tumor grade, specific histology, tumor location, number of positive lymph nodes, and metastases. Each tumor stage was coded as described by the AJCC fifth and sixth editions according to the TNM stage organization for each edition (T1 = tumor invades submucosa; T2 = tumor invades muscularis propria; T3 = tumor invades through the muscularis propria into the subserosa or into nonperitonealized pericolic tissues; T4 = tumor directly invades other organs or structures and/or perforates visceral peritoneum; N0 = no regional lymph node metastasis; N1 = metastasis to one to three regional lymph nodes; N2 = metastasis to four or more regional lymph nodes; M0 = no distant metastasis; M1 = distant metastasis). TNM stage was determined by SEER’s “extent of disease” (for T stage and M stage) and “number of positive [lymph] nodes” (for N stage) coding schemes. All patients were included in both analyses of survival for both staging systems. Tumor grade was categorized as low grade (well or moderately differentiated) and high grade (poorly differentiated, anaplastic, or undifferentiated). Tumor location was categorized as right (cecum, ascending colon, hepatic flexure), transverse, left (splenic flexure, descending colon), and sigmoid colon. The numbers of positive lymph nodes were categorized and examined.
Survival and Statistical Analyses
Kaplan–Meier analyses were performed to determine and to compare overall and stage-specific 5-year survivals for stages defined by both the fifth and sixth edition staging systems. Colon cancer–specific survival (as opposed to observed survival or survival from any cause of death) was determined to obtain the most specific survival associated with colon cancer. In additional analyses, we used AJCC fifth edition staging as a base and stratified data into additional stages by other potential prognostic factors. For this analysis, we used the Kaplan–Meier method and the fifth edition staging system and then stratified the stages by tumor grade, specific histologic type, tumor location, and number of positive lymph nodes. We also evaluated a proposed system of staging that separated the N stage into stages N1 (one to three positive lymph nodes), N2 (four or five positive lymph nodes), N3 (six to eight positive lymph nodes), and N4 (nine or more positive lymph nodes) (Table 2). Because there is no consensus in the literature regarding potential further separation of N stage, we used data on the number of positive lymph nodes for all patients evaluated and analyzed these data by use of a histogram, and broke the data into reasonable groups based on the number of patients with each number of lymph nodes and the dropoffs noted on the histogram after three lymph nodes, five lymph nodes, or eight lymph nodes.
All statistical analyses used SAS version 8.02 (SAS Institute, Cary, NC) or Stata Intercooled version 7.0 (Stata, College Station, TX). P values for survival curves were determined from the Kaplan–Meier survival curves by use of the log-rank test. Those values less than .05 were considered statistically significant. All statistical tests were two-sided.
Results
Demographics
Data from a total of 119 363 patients with colon adenocarcinoma were evaluated. Mean age (± standard deviation) for the cohort was 71.1 ± 12.6 years. Females represented 51.6% of the group, and 54.4% of patients were married at the time of diagnosis. The overall racial and/or ethnic distribution was 77.0% whites, 9.7% blacks, 5.4% Hispanics, 5.9% Asians, and 2.0% other.
Survival by AJCC Fifth and Sixth Edition Staging
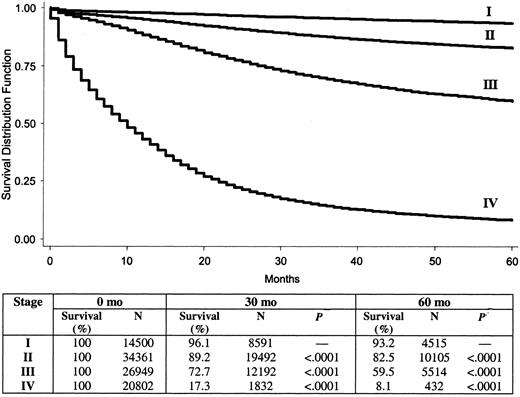
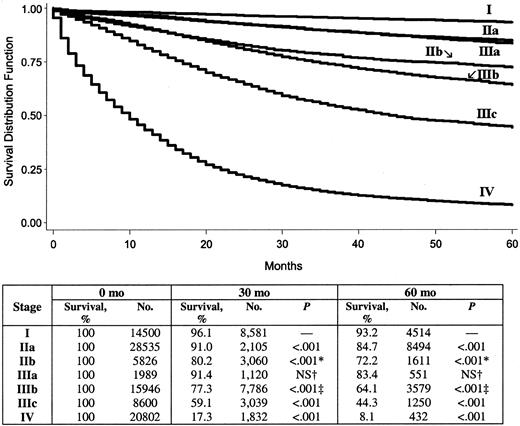
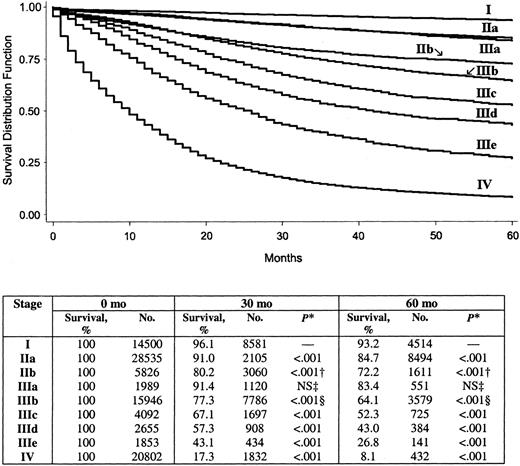
Overall 5-year colon cancer–specific survival for the entire cohort was 65.2%. By use of stages defined by the AJCC fifth edition system, 5-year colon cancer-specific survival by stage was 93.2% for stage I, 82.5% for stage II, 59.5% for stage III, and 8.1% for stage IV. By use of stages defined by the revised AJCC sixth edition system, 5-year colon cancer–specific survival by stage was 93.2% for stage I, 84.7% for stage IIa, 72.2% for stage IIb, 83.4% for stage IIIa, 64.1% for stage IIIb, 44.3% for stage IIIc, and 8.1% for stage IV. The survival analysis for the cohort using the fifth edition staging system is shown in Fig. 1, and that using the sixth edition staging system is in Fig. 2. It is noteworthy that, as defined by the new sixth edition system, stage IIIa colon cancer (83.4%) has a statistically significantly better prognosis than stage IIb colon cancer (72.2%) (P<.001).
Survival by Tumor Grade
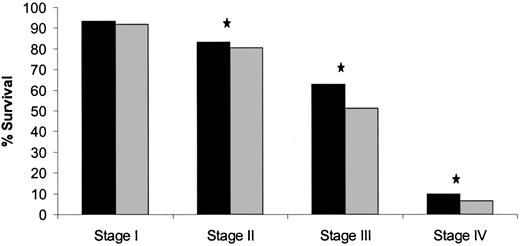
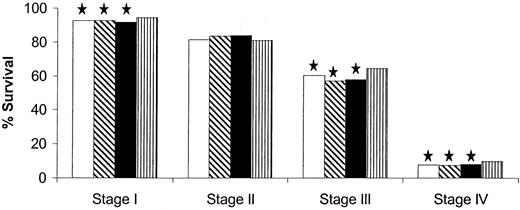
We next used colon cancer stages as defined by the AJCC fifth edition system and stratified data in each stage further by other factors to assess their prognostic value. Among all patients evaluated in the cohort, 67.8% (n = 81 493) had low-grade tumors, 19.4% (n = 23 287) had high-grade tumors, and 12.8% (n = 15 343) had tumors whose grade was unknown. For those patients whose tumor grade (high versus low) was known (n = 104 780), tumor grade was statistically significantly associated with survival in stages II, III, and IV, but not in stage I (Fig. 3).
Survival by Histologic Subtype
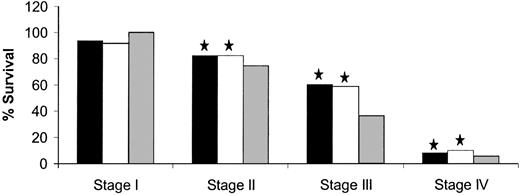
Among patients in the entire cohort, 87.4% had adenocarcinomas, 11.6% had mucinous adenocarcinomas, and 1.0% had signet ring cell carcinomas. Among the entire cohort, a worse 5-year survival was statistically significantly associated with signet ring cell carcinomas (36.0%) than with adenocarcinomas (65.9%) (P<.001) or with mucinous adenocarcinomas (61.8%) (P<.001). When we further stratified data in each stage (as defined by the fifth edition system) by histologic subtype, we observed similar survival distributions in stages II, III, and IV, but not in stage I (Fig. 4). For example, in stage III, the 5-year survival was 36.6% for signet ring cell carcinomas, 60.1% for adenocarcinomas (P<.001), and 58.7% for mucinous adenocarcinomas (P<.001). For stage I, however, the 5-year survival was 100.0% for signet ring cell carcinomas, 93.3% for adenocarcinomas, and 92.0% for mucinous adenocarcinomas; these values were not statistically significantly different from each other.
Survival by Tumor Location
Among patients in the entire cohort, 44.6% had tumors in the right colon, 9.4% had tumors in the transverse colon, 10.4% had tumors in the left colon, 31.6% had tumors in the sigmoid colon, and 4.0% had tumors whose location was unknown. Among the overall cohort, a better 5-year survival was statistically significantly associated with tumors located in the sigmoid colon (69.8%) than with tumors located in the right colon (63.7%) (P<.001), in the transverse colon (65.0%) (P<.001), and in the left colon (65.1%) (P<.001). When we further stratified each stage (as defined by the fifth edition system) by these tumor locations, we observed similar survival distributions in stages I, III, and IV, but not in stage II (Fig. 5). For example, in stage III, 5-year survival was 64.3% for sigmoid lesions, 57.0% for right-colon lesions (P<.001), 57.9% for transverse (P<.001), and 60.2% for left-colon lesions (P<.001), whereas in stage II, 5-year survival was 83.6% and 83.7%, respectively, for right- and transverse-colon lesions, 81.5% for the left colon, and 80.7% for sigmoid lesions.
Number of Positive Lymph Nodes and Proposed Staging System
Among patients in the entire cohort, 32.5% had positive lymph nodes. When we used a histogram analysis of the number of positive lymph nodes, we found that the N stage could be stratified into the following four categories: N1 (one to three positive lymph nodes), N2 (four or five positive lymph nodes), N3 (six to eight positive lymph nodes), and N4 (nine or more positive lymph nodes). We used the proposed N stages in combination with the AJCC sixth edition staging system as a new staging system (Table 2). In this new system, stages I, IIa, IIb, IIIa, and IIIb are the same as corresponding stages in the sixth edition system, but the new stages IIIc, IIId, and IIIe are stratified by categories N2, N3, and N4, respectively, as defined above. The 5-year survival by these proposed stages is 93.2% for stage I, 84.7% for stage IIa, 72.2% for stage IIb, 83.4% for stage IIIa, 64.1% for stage IIIb, 52.3% for stage IIIc, 43.0% for stage IIId, 26.8% for stage IIIe, and 8.1% for stage IV. Corresponding Kaplan–Meier survival curves for this system are shown in Fig. 6.
Discussion
The TNM system for staging cancer was developed in the 1940s (4) and since then has become an important and dynamic part of our cancer language. Many physicians also still continue to use other staging systems with long-standing histories, the Dukes’ staging system (5) and the Astler–Coller (6) and Kirklin (7) modifications of the Dukes’ staging system. For the evaluation of administrative data samples and/or data from cancer registries, however, a unified language for colorectal cancer staging is crucial. These large, population-based datasets are valuable assets in that they provide very large data samples of patients to study and are often population based so that they more clearly reflect the nation as a whole. However, they are useful for cancer research only if a clear and consistent staging system is used (8).
We used national population-based cancer data from the SEER cancer registry to determine the 5-year survival rates for colon cancer with stages defined by both the AJCC fifth edition and the revised AJCC sixth edition staging systems. The fifth edition system separated patients with colon cancer into four stages, with somewhat wide spacing between survival curves, particularly around stages II and III. The sixth edition system for colon cancer stratified stages II and III into two and three subsets, respectively, thereby filling in some of the gaps in the survival curves.
An interesting finding of this study is that, with the AJCC sixth edition staging system, stage IIIa disease appears to be associated with a statistically significantly improved survival compared with that of stage IIb disease. This finding may reflect the National Institutes of Health (NIH) 1990 Consensus Conference guidelines (9) for colorectal cancer that recommend adjuvant chemotherapy for stage III colon cancer patients but do not recommend adjuvant chemotherapy for stage II patients. That is, treatment practices that followed these guidelines may contribute to the finding that early stage III patients (i.e., stage IIIa) had better survival rates than late stage II patients (i.e., stage IIb). Unfortunately, we could not analyze this issue further with SEER data because information about chemotherapy is not available in the SEER database. Another possible explanation for the finding is misclassification of T4N1 (stage IIIa) tumors as T4N0 (stage IIb) tumors. If misclassification occurred, stage IIb patients may also have had micrometastases, which, if untreated, may have been associated with decreased survival. Alternatively, stage IIb tumors may simply be more aggressive tumors, which have a poorer prognosis. Finally, stage IIb tumors may not have received proper en bloc surgical resection, so that residual disease was not excised or an incision went through the tumor, both of which have been associated with a worse prognosis (10).
Since publication of the NIH guidelines (9), there has been some controversy about whether stage II patients, or a certain subset of them, should receive chemotherapy. Recent studies have identified potential high-risk stage II patients who may benefit from such treatment (11,12). Given our findings, patients with stage IIb (i.e., T4N0) colon cancer appear to be one group that may benefit from adjuvant chemotherapy.
Another aim of this study was to evaluate other factors that could be incorporated into a new colon cancer staging system as prognostic factors. We studied four variables (grade, histologic subtypes, tumor location, and number of positive lymph nodes) and applied them to data separated into stages as defined by the AJCC fifth edition staging system. We found, similar to previous results (13), statistically significant differences in survival between patients with high-grade tumors and patients with low-grade tumors. These differences were observed for all stages as defined by the AJCC fifth edition system, except stage I. The International Union Against Cancer and the AJCC are constantly reevaluating the effectiveness of the TNM system and of various options to it. Grade may be a factor to investigate further for colon cancer staging systems (14).
We also evaluated the association of histologic type of colon cancer and tumor location with survival. We found that signet ring cell carcinomas were statistically significantly associated with worse survival than adenocarcinomas and mucinous adenocarcinomas. However, only 1% of the overall cohort has signet ring cell carcinomas. Consequently, histologic subtype is probably not a useful addition to staging systems. When we investigated tumor location, we found that sigmoid colon tumors were associated with statistically significantly better survival than tumors of the right, transverse, and left colon. However, the differences, although statistically significant, were small and are not likely to be clinically significant.
The fourth factor we evaluated, the number of positive lymph nodes, is already part of the TNM classification. However, our proposed scheme would use four N stages rather than two. When the survival curves generated by use of this proposed system were examined, we found that the curves provided more detailed (i.e., stratified) prognostic information than did current systems. Furthermore, because the AJCC and the International Union Against Cancer have recommended that a minimum of 12 lymph nodes be examined for proper staging of stage III patients (8,15–17), it is not unreasonable to stratify the N stage into the four stages that we propose, which stratify up to nine or more positive lymph nodes. Although the increased stratification of a staging system may not necessarily lead to different treatments (e.g., stage IIIa versus IIIb), it does provide important prognostic information that may help future clinical trials, improve the evidence-based literature, and provide more information to health care providers and patients. Several other potential staging stratifications have been created for stratifying stage III tumors (8), and ours is another option.
Although this is a large population-based study evaluating survival and stages for colon cancer defined by the AJCC fifth edition staging system compared with stages defined by the new sixth edition system, it has several potential limitations. First, although the SEER registry maintains stringent quality control measures to prevent coding errors, miscoding and inaccurate data may be present. SEER, however, does uphold several measures to ensure accuracy and maintains the highest level of certification of data quality and completeness, as reported by the North American Association of Central Cancer Registries (18). Second, the SEER registry does not contain all clinically relevant treatment data, the most important for our study being the receipt of chemotherapy. Data on treatment would be helpful in analyzing and understanding some of our results.
Nevertheless, our use of the population-based SEER registry also provided benefits to our study. First, we were able to analyze data from 119 363 patients diagnosed with colon adenocarcinoma over a 10-year period. Second, SEER is a national sample representing approximately 14% of the U.S. population (3). As such, our finding should generalize to the overall population of the United States.
In summary, this work is one of the largest studies to date evaluating the new AJCC sixth edition staging system for colon cancer. We found that the new AJCC sixth edition staging system for colon cancer results in better estimates of survival by providing more substages than the fifth edition system. In addition, we demonstrate that other factors may be used to improve colon cancer staging, such as tumor grade and additional stratification of the number of positive lymph nodes. It is unclear why stage IIIa patients appear to have better survival than that of stage IIb, but this finding brings up the important and debated question of whether patients with stage II colon cancer should receive adjuvant therapy. Further studies to determine whether stage IIb patients would benefit from receiving chemotherapy routinely may be indicated.
Five-year survival by American Joint Committee on Cancer fifth edition system stages I–IV. P value determined with the log-rank test refers to the corresponding stage and the stage in the row above. All statistical tests were two-sided.
Five-year survival by the American Joint Committee on Cancer sixth edition system stages I–IV. P value determined by the log-rank test refers to the corresponding stage and the stage in the row above, unless otherwise indicated. All statistical tests were two-sided. * = IIIa versus IIb; † = IIa versus IIIa; ‡ = IIb versus IIIb; NS = not statistically significant.
Five-year survival for American Joint Committee on Cancer fifth edition by grade. Solid bars, low-grade tumors; shaded bars, high-grade tumors. Star, P<.001, log-rank test. All statistical tests were two-sided.
Five-year survival for American Joint Committee on Cancer fifth edition by histologic subtype. Solid bars, adeno; open bars, mucinous; shaded bars, signet cell. Star, P<.001, log-rank test, compared with signet cell carcinoma. All statistical tests were two-sided.
Five-year survival for American Joint Committee on Cancer fifth edition by tumor location. Open bars, left; diagonal-hatched bars, right; solid bars, transverse; vertical-hatched bars, sigmoid. Star, P<.001, log-rank test, compared with sigmoid colon. All statistical tests were two-sided.
Survival by American Joint Committee on Cancer sixth edition staging with proposed lymph node (N) stages. *, P values determined by the log-rank test refers to the corresponding stage and the stage in the row above, unless otherwise indicated. † = IIIa versus IIb; ‡ = IIa versus IIIa; § = IIb versus IIIb; NS = not statistically significant. All statistical tests were two-sided.
Stages as defined by the American Joint Committee on Cancer (AJCC) fifth and sixth edition staging systems*
| Staging system . | T stage . | N stage . | M stage . |
|---|---|---|---|
| AJCC fifth edition | |||
| I | T1 or T2 | N0 | M0 |
| II | T3 or T4 | N0 | M0 |
| III | Any T | N1 | M0 |
| IV | Any T | Any N | M1 |
| AJCC sixth edition | |||
| I | T1 or T2 | N0 | M0 |
| IIa | T3 | N0 | M0 |
| IIb | T4 | N0 | M0 |
| IIIa | T1 or T2 | N1 | M0 |
| IIIb | T3 or T4 | N1 | M0 |
| IIIc | Any T | N2 | M0 |
| IV | Any T | Any N | M1 |
| Staging system . | T stage . | N stage . | M stage . |
|---|---|---|---|
| AJCC fifth edition | |||
| I | T1 or T2 | N0 | M0 |
| II | T3 or T4 | N0 | M0 |
| III | Any T | N1 | M0 |
| IV | Any T | Any N | M1 |
| AJCC sixth edition | |||
| I | T1 or T2 | N0 | M0 |
| IIa | T3 | N0 | M0 |
| IIb | T4 | N0 | M0 |
| IIIa | T1 or T2 | N1 | M0 |
| IIIb | T3 or T4 | N1 | M0 |
| IIIc | Any T | N2 | M0 |
| IV | Any T | Any N | M1 |
T1 = tumor invades submucosa; T2 = tumor invades muscularis propria; T3 = tumor invades through the muscularis propria into the subserosa or into nonperitonealized pericolic tissues; T4 = tumor directly invades other organs or structures and/or perforates visceral peritoneum; N0 = no regional lymph node metastasis; N1 = metastasis to one to three regional lymph nodes; N2 = metastasis to four or more regional lymph nodes; M0 = no distant metastasis; M1 = distant metastasis.
Stages as defined by the American Joint Committee on Cancer (AJCC) fifth and sixth edition staging systems*
| Staging system . | T stage . | N stage . | M stage . |
|---|---|---|---|
| AJCC fifth edition | |||
| I | T1 or T2 | N0 | M0 |
| II | T3 or T4 | N0 | M0 |
| III | Any T | N1 | M0 |
| IV | Any T | Any N | M1 |
| AJCC sixth edition | |||
| I | T1 or T2 | N0 | M0 |
| IIa | T3 | N0 | M0 |
| IIb | T4 | N0 | M0 |
| IIIa | T1 or T2 | N1 | M0 |
| IIIb | T3 or T4 | N1 | M0 |
| IIIc | Any T | N2 | M0 |
| IV | Any T | Any N | M1 |
| Staging system . | T stage . | N stage . | M stage . |
|---|---|---|---|
| AJCC fifth edition | |||
| I | T1 or T2 | N0 | M0 |
| II | T3 or T4 | N0 | M0 |
| III | Any T | N1 | M0 |
| IV | Any T | Any N | M1 |
| AJCC sixth edition | |||
| I | T1 or T2 | N0 | M0 |
| IIa | T3 | N0 | M0 |
| IIb | T4 | N0 | M0 |
| IIIa | T1 or T2 | N1 | M0 |
| IIIb | T3 or T4 | N1 | M0 |
| IIIc | Any T | N2 | M0 |
| IV | Any T | Any N | M1 |
T1 = tumor invades submucosa; T2 = tumor invades muscularis propria; T3 = tumor invades through the muscularis propria into the subserosa or into nonperitonealized pericolic tissues; T4 = tumor directly invades other organs or structures and/or perforates visceral peritoneum; N0 = no regional lymph node metastasis; N1 = metastasis to one to three regional lymph nodes; N2 = metastasis to four or more regional lymph nodes; M0 = no distant metastasis; M1 = distant metastasis.
Proposed staging system*
| Stage . | T stage . | N stage . | M stage . |
|---|---|---|---|
| I | T1 or T2 | N0 | M0 |
| IIa | T3 | N0 | M0 |
| IIb | T4 | N0 | M0 |
| IIIa | T1 or T2 | N1 | M0 |
| IIIb | T3 or T4 | N1 | M0 |
| IIIc | Any T | N2 | M0 |
| IIId | Any T | N3 | M0 |
| IIIc | Any T | N4 | M0 |
| IV | Any T | Any N | M1 |
| Stage . | T stage . | N stage . | M stage . |
|---|---|---|---|
| I | T1 or T2 | N0 | M0 |
| IIa | T3 | N0 | M0 |
| IIb | T4 | N0 | M0 |
| IIIa | T1 or T2 | N1 | M0 |
| IIIb | T3 or T4 | N1 | M0 |
| IIIc | Any T | N2 | M0 |
| IIId | Any T | N3 | M0 |
| IIIc | Any T | N4 | M0 |
| IV | Any T | Any N | M1 |
Stages I, IIa, IIb, IIIa, and IIIb are the same as in the American Joint Committee on Cancer sixth edition system. T1 = tumor invades submucosa; T2 = tumor invades muscularis propria; T3 = tumor invades through the muscularis propria into the subscrosa or into nonperitonealized pericolic tissues; T4 = tumor directly invades other organs or structures and/or perforates visceral peritoneum; N0 = no positive lymph nodes; N1 = one to three positive lymph nodes; N2 = four or five positive lymph nodes; N3 = six to eight positive lymph nodes; N4 = nine or more positive lymph nodes; M0 = no distant metastasis; M1 = distant metastasis.
Proposed staging system*
| Stage . | T stage . | N stage . | M stage . |
|---|---|---|---|
| I | T1 or T2 | N0 | M0 |
| IIa | T3 | N0 | M0 |
| IIb | T4 | N0 | M0 |
| IIIa | T1 or T2 | N1 | M0 |
| IIIb | T3 or T4 | N1 | M0 |
| IIIc | Any T | N2 | M0 |
| IIId | Any T | N3 | M0 |
| IIIc | Any T | N4 | M0 |
| IV | Any T | Any N | M1 |
| Stage . | T stage . | N stage . | M stage . |
|---|---|---|---|
| I | T1 or T2 | N0 | M0 |
| IIa | T3 | N0 | M0 |
| IIb | T4 | N0 | M0 |
| IIIa | T1 or T2 | N1 | M0 |
| IIIb | T3 or T4 | N1 | M0 |
| IIIc | Any T | N2 | M0 |
| IIId | Any T | N3 | M0 |
| IIIc | Any T | N4 | M0 |
| IV | Any T | Any N | M1 |
Stages I, IIa, IIb, IIIa, and IIIb are the same as in the American Joint Committee on Cancer sixth edition system. T1 = tumor invades submucosa; T2 = tumor invades muscularis propria; T3 = tumor invades through the muscularis propria into the subscrosa or into nonperitonealized pericolic tissues; T4 = tumor directly invades other organs or structures and/or perforates visceral peritoneum; N0 = no positive lymph nodes; N1 = one to three positive lymph nodes; N2 = four or five positive lymph nodes; N3 = six to eight positive lymph nodes; N4 = nine or more positive lymph nodes; M0 = no distant metastasis; M1 = distant metastasis.
1Editor’s note: SEER is a set of geographically defined, population-based, central cancer registries in the United States, operated by local nonprofit organizations under contract to the National Cancer Institute (NCI). Registry data are submitted electronically without personal identifiers to the NCI on a biannual basis, and the NCI makes the data available to the public for scientific research.
References
American Joint Committee on Cancer. Missions and objectives. Available at: http://www.cancerstaging.org. [Last accessed: August 6, 2004.]
Surveillance, Epidemiology, and End Results (SEER) Program Public-Use Data (1973-2000), National Cancer Institute, DCCPS, Surveillance Research Program, Cancer Statistics Branch, released April 2002, based on the November 2001 submission. Available at: http://www.seer.cancer.gov. [Last accessed: August 6,
Denoix PF, Schwartz D. [General rules for classification of cancers and presentation of the therapeutic results.]
Dukes CE, Bussey HJ. The spread of rectal cancer and its effect on prognosis.
Astler VB, Coller FA. The prognostic significance of direct extension of carcinoma of the colon and rectum.
Kirklin JW, Dockerty MD, Waugh JW. The role of the peritoneal reflection in the prognosis of carcinoma of the rectum and sigmoid colon.
Greene FL. TNM staging for malignancies of the digestive tract: 2003 changes and beyond.
NIH consensus conference. Adjuvant therapy for patients with colon and rectal cancer.
Nelson H, Petrelli N, Carlin A, Couture J, Fleshman J, Guillem J, et al. Guidelines 2000 for colon and rectal cancer surgery.
Mulcahy HE, Toner M, Patchett SE, Daly L, O’Donoghue DP. Identifying stage B colorectal cancer patients at high risk of tumor recurrence and death.
Merkel S, Wein A, Gunther K, Papadopoulos T, Hohenberger W, Hermanek P. High-risk groups of patients with stage II colon carcinoma.
Compton CC, Fielding LP, Burgart LJ, Conley B, Cooper HS, Hamilton SR, et al. Prognostic factors in colorectal cancer. College of American Pathologists Consensus Statement 1999.
Gospodarowicz MK, Miller D, Groome PA, Greene FL, Logan PA, Sobin LH. The process for continuous improvement of the TNM classification.
Wong JH, Severino R, Honnebier MB, Tom P, Namiki TS. Number of nodes examined and staging accuracy in colorectal carcinoma.
Swanson RS, Compton CC, Stewart AK, Bland KI. The prognosis of T3N0 colon cancer is dependent on the number of lymph nodes examined.
Goldstein NS, Sanford W, Coffey M, Layfield LJ. Lymph node recovery from colorectal resection specimens removed for adenocarcinoma. Trends over time and a recommendation for a minimum number of lymph nodes to be recovered.
North American Association of Central Cancer Registries. Available at: http://www.naaccr.org. [Last accessed: August 6,