Abstract
Background/Objectives:
A significant proportion of Crohn’s disease (CD) patients receiving infliximab (IFX) maintenance therapy show loss of responsiveness despite a good initial response. The factors other than immunomodulators that prevent IFX dose escalation have yet to be fully elucidated. This study was performed to identify clinical factors or concomitant therapies associated with sustained response to IFX.
Subjects/Methods:
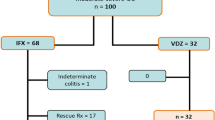
Seventy-four consecutive CD patients who had successful IFX induction therapy between 2002 and 2010 underwent IFX maintenance therapy. Patients showing loss of response to IFX were treated with IFX intensification therapy. Factors involved in the sustained response to IFX were investigated retrospectively.
Results:
After a median follow-up of 85 weeks, loss of response to IFX was observed in 30 (40.5%) cases. On logistic regression analysis, concomitant use of enteral nutrition (EN) therapy (elemental and/or polymeric formulas) was identified as an independent factor associated with sustained response to IFX. Receiver operating characteristic curve analysis indicated a cutoff value of 600 kcal/day. We divided the patients into the ‘EN group’ (⩾600 kcal/day) and ‘control group’ (<600 kcal/day). The cumulative number of loss of response was significantly lower in the EN group (odds ratio: 0.23, P=0.0043). Kaplan–Meier analysis confirmed the significantly lower rate of loss of response in the EN group (P=0.013). Multivariate hazard ratio was 0.37 (P=0.025). Type of EN formula did not affect the results.
Conclusions:
Concomitant use of EN ⩾600 kcal/day is likely to yield a sustained response to IFX in CD patients.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Khor B, Gardet A, Xavier RJ . Genetics and pathogenesis of inflammatory bowel disease. Nature 2011; 474: 307–317.
Maloy KJ, Powrie F . Intestinal homeostasis and its breakdown in inflammatory bowel disease. Nature 2011; 474: 298–306.
Strober W, Fuss IJ . Proinflammatory cytokines in the pathogenesis of inflammatory bowel diseases. Gastroenterology 2011; 140: 1756–1767.
Burger D, Travis S . Conventional medical management of inflammatory bowel disease. Gastroenterology 2011; 140: 1827–1837. ; e1822.
Danese S, Colombel JF, Reinisch W, Rutgeerts PJ . Review article: infliximab for Crohn’s disease treatment—shifting therapeutic strategies after 10 years of clinical experience. Aliment Pharmacol Ther 2011; 33: 857–869.
Targan SR, Hanauer SB, van Deventer SJ, Mayer L, Present DH, Braakman T et al. A short-term study of chimeric monoclonal antibody cA2 to tumor necrosis factor alpha for Crohn’s disease. Crohn’s Disease cA2 Study Group. N Engl J Med 1997; 337: 1029–1035.
Hanauer SB, Feagan BG, Lichtenstein GR, Mayer LF, Schreiber S, Colombel JF et al. Maintenance infliximab for Crohn’s disease: the ACCENT I randomised trial. Lancet 2002; 359: 1541–1549.
Sands BE, Anderson FH, Bernstein CN, Chey WY, Feagan BG, Fedorak RN et al. Infliximab maintenance therapy for fistulizing Crohn’s disease. N Engl J Med 2004; 350: 876–885.
Gisbert JP, Panes J . Loss of response and requirement of infliximab dose intensification in Crohn’s disease: a review. Am J Gastroenterol 2009; 104: 760–767.
Ben-Horin S, Chowers Y . Review article: loss of response to anti-TNF treatments in Crohn’s disease. Aliment Pharmacol Ther 2011; 33: 987–995.
Danese S, Fiorino G, Reinisch W . Review article: Causative factors and the clinical management of patients with Crohn’s disease who lose response to anti-TNF-alpha therapy. Aliment Pharmacol Ther 2011; 34: 1–10.
Colombel JF, Sandborn WJ, Reinisch W, Mantzaris GJ, Kornbluth A, Rachmilewitz D et al. Infliximab, azathioprine, or combination therapy for Crohn’s disease. N Engl J Med 2010; 362: 1383–1395.
Vermeire S, Noman M, Van Assche G, Baert F, D’Haens G, Rutgeerts P . Effectiveness of concomitant immunosuppressive therapy in suppressing the formation of antibodies to infliximab in Crohn’s disease. Gut 2007; 56: 1226–1231.
Schnitzler F, Fidder H, Ferrante M, Noman M, Arijs I, Van Assche G et al. Long-term outcome of treatment with infliximab in 614 patients with Crohn’s disease: results from a single-centre cohort. Gut 2009; 58: 492–500.
Kopylov U, Mantzaris GJ, Katsanos KH, Reenaers C, Ellul P, Rahier JF et al. The efficacy of shortening the dosing interval to once every six weeks in Crohn’s patients losing response to maintenance dose of infliximab. Aliment Pharmacol Ther 2011; 33: 349–357.
Yao T, Matsui T, Hiwatashi N . Crohn’s disease in Japan: diagnostic criteria and epidemiology. Dis Colon Rectum 2000; 43: S85–S93.
Tanaka T, Takahama K, Kimura T, Mizuno T, Nagasaka M, Iwata K et al. Effect of concurrent elemental diet on infliximab treatment for Crohn’s disease. J Gastroenterol Hepatol 2006; 21: 1143–1149.
Goh J, O’Morain CA . Review article: nutrition and adult inflammatory bowel disease. Aliment Pharmacol Ther 2003; 17: 307–320.
Griffiths AM . Enteral nutrition in the management of Crohn’s disease. J Parenter Enteral Nutr 2005; 29: S108–S112. discussion S112–107, S184–108.
Lochs H . Enteral nutrition-the new maintenance therapy in Crohn’s disease? Inflamm Bowel Dis 2007; 13: 1581–1582.
Rigaud D, Cosnes J, Le Quintrec Y, Rene E, Gendre JP, Mignon M . Controlled trial comparing two types of enteral nutrition in treatment of active Crohn’s disease: elemental versus polymeric diet. Gut 1991; 32: 1492–1497.
Verma S, Brown S, Kirkwood B, Giaffer MH . Polymeric versus elemental diet as primary treatment in active Crohn’s disease: a randomized, double-blind trial. Am J Gastroenterol 2000; 95: 735–739.
Ameho CK, Adjei AA, Harrison EK, Takeshita K, Morioka T, Arakaki Y et al. Prophylactic effect of dietary glutamine supplementation on interleukin 8 and tumour necrosis factor alpha production in trinitrobenzene sulphonic acid induced colitis. Gut 1997; 41: 487–493.
Tsune I, Ikejima K, Hirose M, Yoshikawa M, Enomoto N, Takei Y et al. Dietary glycine prevents chemical-induced experimental colitis in the rat. Gastroenterology 2003; 125: 775–785.
Andou A, Hisamatsu T, Okamoto S, Chinen H, Kamada N, Kobayashi T et al. Dietary histidine ameliorates murine colitis by inhibition of proinflammatory cytokine production from macrophages. Gastroenterology 2009; 136: 564–574. ; e562.
Johnson T, Macdonald S, Hill SM, Thomas A, Murphy MS . Treatment of active Crohn’s disease in children using partial enteral nutrition with liquid formula: a randomised controlled trial. Gut 2006; 55: 356–361.
Korelitz BI . The role of liquid diet in the management of small bowel Crohn’s disease. Inflamm Bowel Dis 2000; 6: 66–67. ; discussion 68–69.
Hirakawa H, Fukuda Y, Tanida N, Hosomi M, Shimoyama T . Home elemental enteral hyperalimentation (HEEH) for the maintenance of remission in patients with Crohn’s disease. Gastroenterol Jpn 1993; 28: 379–384.
Fukuda Y, Kosaka T, Okui M, Hirakawa H, Shimoyama T . Efficacy of nutritional therapy for active Crohn’s disease. J Gastroenterol 1995; 30 (Suppl 8), 83–87.
Takagi S, Utsunomiya K, Kuriyama S, Yokoyama H, Takahashi S, Iwabuchi M et al. Effectiveness of an ‘half elemental diet’ as maintenance therapy for Crohn’s disease: a randomized-controlled trial. Aliment Pharmacol Ther 2006; 24: 1333–1340.
Acknowledgements
We thank Atsushi Isono, Hirotsugu Uehara, Yasutaka Kato, Kiyoshi Furukawa, Kazuki Hatakeyama, Shin-ichiro Takeda, Yasushi Mandai, Tomoo Makita, Yoshie Takahashi and Yuhoko Furuya (Department of Gastroenterology and Hepatology, Chiba University Hospital, Japan) for their clinical assistance.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Sazuka, S., Katsuno, T., Nakagawa, T. et al. Concomitant use of enteral nutrition therapy is associated with sustained response to infliximab in patients with Crohn’s disease. Eur J Clin Nutr 66, 1219–1223 (2012). https://doi.org/10.1038/ejcn.2012.120
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ejcn.2012.120
Keywords
This article is cited by
-
Contemporary Dietary Therapies in Inflammatory Bowel Disease
Current Treatment Options in Pediatrics (2021)
-
Efficacy of enteral nutrition in patients with Crohn’s disease on maintenance anti-TNF-alpha antibody therapy: a meta-analysis
Journal of Gastroenterology (2020)
-
The Role of Diet in Inflammatory Bowel Disease
Current Gastroenterology Reports (2017)
-
Clinical efficacy of adalimumab in Crohn’s disease: a real practice observational study in Japan
BMC Gastroenterology (2016)
-
Two-Year Outcomes After Exclusive Enteral Nutrition Induction Are Superior to Corticosteroids in Pediatric Crohn’s Disease Treated Early with Thiopurines
Digestive Diseases and Sciences (2015)