Abstract
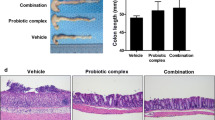
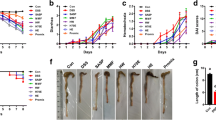
VSL#3 probiotics can be effective on induction and maintenance of the remission of clinical ulcerative colitis. However, the mechanisms are not fully understood. The aim of this study was to examine the effects of VSL#3 probiotics on dextran sulfate sodium (DSS)-induced colitis in rats. Acute colitis was induced by administration of DSS 3.5 % for 7 days in rats. Rats in two groups were treated with either 15 mg VSL#3 or placebo via gastric tube once daily after induction of colitis; rats in other two groups were treated with either the wortmannin (1 mg/kg) via intraperitoneal injection or the wortmannin + VSL#3 after induction of colitis. Anti-inflammatory activity was assessed by myeloperoxidase (MPO) activity. Expression of inflammatory related mediators (iNOS, COX-2, NF-κB, Akt, and p-Akt) and cytokines (TNF-α, IL-6, and IL-10) in colonic tissue were assessed. TNF-α, IL-6, and IL-10 serum levels were also measured. Our results demonstrated that VSL#3 and wortmannin have anti-inflammatory properties by the reduced disease activity index and MPO activity. In addition, administration of VSL#3 and wortmannin for 7 days resulted in a decrease of iNOS, COX-2, NF-κB, TNF-α, IL-6, and p-Akt and an increase of IL-10 expression in colonic tissue. At the same time, administration of VSL#3 and wortmannin resulted in a decrease of TNF-α and IL-6 and an increase of IL-10 serum levels. VSL#3 probiotics therapy exerts the anti-inflammatory activity in rat model of DSS-induced colitis by inhibiting PI3K/Akt and NF-κB pathway.







Similar content being viewed by others
References
Bernstein CN (2010) New insights into IBD epidemiology: are there any lessons for treatment? Dig Dis 28(3):406–410
Fiasse R, Denis MA, Dewit O (2010) Chronic inflammatory bowel disease: Crohn’s disease and ulcerative colitis. J Pharm Belg 1:1–9
Floch MH (2003) Probiotics, irritable bowel syndrome, and inflammatory bowel disease. Curr Treat Options Gastroenterol 6(4):283–288
Leske D, Hoffmann JC (2010) Inflammatory bowel disease: longterm management. MMW Fortschr Med 152(28–30):40–42
Morson BC (1980) Pathology of inflammatory bowel disease. Gastroenterol Jpn 15(2):184–187
Angulo S, Morales A, Danese S, Llacuna L, Masamunt MC, Pultz N, Cifone MG, De Simone C, Delgado S, Vila J, Panes J, Donskey C, Fernandez-Checa JC, Fiocchi C, Sans M (2011) Probiotic sonicates selectively induce mucosal immune cells apoptosis through ceramide generation via neutral sphingomyelinase. PLoS One 6(3):e16953. doi:10.1371/journal.pone.0016953
Yuan H, Ji WS, Wu KX, Jiao JX, Sun LH, Feng YT (2006) Anti-inflammatory effect of diammonium glycyrrhizinate in a rat model of ulcerative colitis. World J Gastroenterol 12(28):4578–4581
Choi SY, Hur SJ, An CS, Jeon YH, Jeoung YJ, Bak JP, Lim BO (2010) Anti-inflammatory effects of Inonotus obliquus in colitis induced by dextran sodium sulfate. J Biomed Biotechnol 2010:943516. doi:10.1155/2010/943516
Islam MS, Murata T, Fujisawa M, Nagasaka R, Ushio H, Bari AM, Hori M, Ozaki H (2008) Anti-inflammatory effects of phytosteryl ferulates in colitis induced by dextran sulphate sodium in mice. Br J Pharmacol 154(4):812–824. doi:10.1038/bjp.2008.137
Oh PS, Lim KT (2006) Plant originated glycoprotein has anti-oxidative and anti-inflammatory effects on dextran sulfate sodium-induced colitis in mouse. J Biomed Sci 13(4):549–560. doi:10.1007/s11373-006-9083-9
Domizio P (1994) Pathology of chronic inflammatory bowel disease in children. Baillieres Clin Gastroenterol 8(1):35–63
Cui HH, Chen CL, Wang JD, Yang YJ, Cun Y, Wu JB, Liu YH, Dan HL, Jian YT, Chen XQ (2004) Effects of probiotic on intestinal mucosa of patients with ulcerative colitis. World J Gastroenterol 10(10):1521–1525
Floch MH, Madsen KK, Jenkins DJ, Guandalini S, Katz JA, Onderdonk A, Walker WA, Fedorak RN, Camilleri M (2006) Recommendations for probiotic use. J Clin Gastroenterol 40(3):275–278
Friswell M, Campbell B, Rhodes J (2010) The role of bacteria in the pathogenesis of inflammatory bowel disease. Gut Liver 4(3):295–306. doi:10.5009/gnl.2010.4.3.295
Guandalini S (2010) Update on the role of probiotics in the therapy of pediatric inflammatory bowel disease. Expert Rev Clin Immunol 6(1):47–54
Haller D, Antoine JM, Bengmark S, Enck P, Rijkers GT, Lenoir-Wijnkoop I (2010) Guidance for substantiating the evidence for beneficial effects of probiotics: probiotics in chronic inflammatory bowel disease and the functional disorder irritable bowel syndrome. J Nutr 140(3):690S–697S. doi:10.3945/jn.109.113746
Hering NA, Schulzke JD (2009) Therapeutic options to modulate barrier defects in inflammatory bowel disease. Dig Dis 27(4):450–454. doi:10.1159/000233283
Williams NT (2010) Probiotics. Am J Health Syst Pharm 67(6):449–458. doi:10.2146/ajhp090168
Huynh HQ, deBruyn J, Guan L, Diaz H, Li M, Girgis S, Turner J, Fedorak R, Madsen K (2009) Probiotic preparation VSL#3 induces remission in children with mild to moderate acute ulcerative colitis: a pilot study. Inflamm Bowel Dis 15(5):760–768. doi:10.1002/ibd.20816
Reiff C, Kelly D (2010) Inflammatory bowel disease, gut bacteria and probiotic therapy. Int J Med Microbiol 300(1):25–33. doi:10.1016/j.ijmm.2009.08.004
Sengul N, Isik S, Aslim B, Ucar G, Demirbag AE (2011) The effect of exopolysaccharide-producing probiotic strains on gut oxidative damage in experimental colitis. Dig Dis Sci 56(3):707–714. doi:10.1007/s10620-010-1362-7
Uronis JM, Arthur JC, Keku T, Fodor A, Carroll IM, Cruz ML, Appleyard CB, Jobin C (2011) Gut microbial diversity is reduced by the probiotic VSL#3 and correlates with decreased TNBS-induced colitis. Inflamm Bowel Dis 17(1):289–297. doi:10.1002/ibd.21366
Wan YM, Zhu YQ, Xia B, Luo J (2010) Probiotic therapy using live combined bifidobacterium, lactobacillus and enterococcus for experimental colitis in rats model. Zhonghua Nei Ke Za Zhi 49(5):418–421
Amit-Romach E, Uni Z, Reifen R (2010) Multistep mechanism of probiotic bacterium, the effect on innate immune system. Mol Nutr Food Res 54(2):277–284. doi:10.1002/mnfr.200800591
Arribas B, Rodriguez-Cabezas ME, Camuesco D, Comalada M, Bailon E, Utrilla P, Nieto A, Concha A, Zarzuelo A, Galvez J (2009) A probiotic strain of Escherichia coli, Nissle 1917, given orally exerts local and systemic anti-inflammatory effects in lipopolysaccharide-induced sepsis in mice. Br J Pharmacol 157(6):1024–1033. doi:10.1111/j.1476-5381.2009.00270.x
Di Giacinto C, Marinaro M, Sanchez M, Strober W, Boirivant M (2005) Probiotics ameliorate recurrent Th1-mediated murine colitis by inducing IL-10 and IL-10-dependent TGF-beta-bearing regulatory cells. J Immunol 174(6):3237–3246
Lee SK, Kim HJ, Chi SG (2010) Saccharomyces boulardii reduced intestinal inflammation in mice model of 2,4,6-trinitrobencene sulfonic acid induced colitis: based on microarray. Korean J Gastroenterol 55(1):33–45
Mohamadzadeh M, Pfeiler EA, Brown JB, Zadeh M, Gramarossa M, Managlia E, Bere P, Sarraj B, Khan MW, Pakanati KC, Ansari MJ, O’Flaherty S, Barrett T, Klaenhammer TR (2011) Regulation of induced colonic inflammation by Lactobacillus acidophilus deficient in lipoteichoic acid. Proc Natl Acad Sci USA 108(Suppl 1):4623–4630. doi:10.1073/pnas.1005066107
Philippe D, Heupel E, Blum-Sperisen S, Riedel CU (2010) Treatment with Bifidobacterium bifidum 17 partially protects mice from Th1-driven inflammation in a chemically induced model of colitis. Int J Food Microbiol. doi:10.1016/j.ijfoodmicro.2010.12.020
Bibiloni R, Fedorak RN, Tannock GW, Madsen KL, Gionchetti P, Campieri M, De Simone C, Sartor RB (2005) VSL#3 probiotic-mixture induces remission in patients with active ulcerative colitis. Am J Gastroenterol 100(7):1539–1546. doi:10.1111/j.1572-0241.2005.41794.x
Chapman TM, Plosker GL, Figgitt DP (2006) VSL#3 probiotic mixture: a review of its use in chronic inflammatory bowel diseases. Drugs 66(10):1371–1387
Guandalini S, Magazzu G, Chiaro A, La Balestra V, Di Nardo G, Gopalan S, Sibal A, Romano C, Canani RB, Lionetti P, Setty M (2010) VSL#3 improves symptoms in children with irritable bowel syndrome: a multicenter, randomized, placebo-controlled, double-blind, crossover study. J Pediatr Gastroenterol Nutr 51(1):24–30. doi:10.1097/MPG.0b013e3181ca4d95
Jackson EL, Hamlin PJ, Ford AC (2011) VSL#3 and remission in active ulcerative colitis: larger studies required. Am J Gastroenterol 106(3):547. doi:10.1038/ajg.2010.451
Yan F, Polk DB (2012) Characterization of a probiotic-derived soluble protein which reveals a mechanism of preventive and treatment effects of probiotics on intestinal inflammatory diseases. Gut Microbes 3(1):25–28. doi:10.4161/gmic.19245
Cooper HS, Murthy SN, Shah RS, Sedergran DJ (1993) Clinicopathologic study of dextran sulfate sodium experimental murine colitis. Lab Invest 69(2):238–249
Rijcken EM, Laukoetter MG, Anthoni C, Meier S, Mennigen R, Spiegel HU, Bruewer M, Senninger N, Vestweber D, Krieglstein CF (2004) Immunoblockade of PSGL-1 attenuates established experimental murine colitis by reduction of leukocyte rolling. Am J Physiol Gastrointest Liver Physiol 287(1):G115–G124. doi:10.1152/ajpgi.00207.2003
Fitzpatrick LR, Hertzog KL, Quatse AL, Koltun WA, Small JS, Vrana K (2007) Effects of the probiotic formulation VSL#3 on colitis in weanling rats. J Pediatr Gastroenterol Nutr 44(5):561–570. doi:10.1097/MPG.0b013e31803bda51
Bjorck S, Jennische E, Dahlstrom A, Ahlman H (1997) Influence of topical rectal application of drugs on dextran sulfate-induced colitis in rats. Dig Dis Sci 42(4):824–832
Bailon E, Comalada M, Roman J, Michelena P, Ramis I, Merlos M, Nieto A, Concha A, Zarzuelo A, Galvez J (2008) UR-1505, a salicylate able to selectively block T-cell activation, shows intestinal anti-inflammatory activity in the chronic phase of the DSS model of rat colitis. Inflamm Bowel Dis 14(7):888–897. doi:10.1002/ibd.20381
Borjesson L, Aldenborg F, Delbro DS (2001) Functional effects of dextran sulphate sodium (DSS) treatment on the longitudinal muscle of rat distal colon. J Auton Pharmacol 21(3):121–129
Gaudier E, Michel C, Segain JP, Cherbut C, Hoebler C (2005) The VSL# 3 probiotic mixture modifies microflora but does not heal chronic dextran-sodium sulfate-induced colitis or reinforce the mucus barrier in mice. J Nutr 135(12):2753–2761
Babbs CF (1992) Oxygen radicals in ulcerative colitis. Free Radic Biol Med 13(2):169–181
Sandborn WJ, Targan SR (2002) Biologic therapy of inflammatory bowel disease. Gastroenterology 122(6):1592–1608
Aktan F (2004) iNOS-mediated nitric oxide production and its regulation. Life Sci 75(6):639–653. doi:10.1016/j.lfs.2003.10.042
Krieglstein CF, Cerwinka WH, Laroux FS, Salter JW, Russell JM, Schuermann G, Grisham MB, Ross CR, Granger DN (2001) Regulation of murine intestinal inflammation by reactive metabolites of oxygen and nitrogen: divergent roles of superoxide and nitric oxide. J Exp Med 194(9):1207–1218
Menchen L, Colon AL, Madrigal JL, Beltran L, Botella S, Lizasoain I, Leza JC, Moro MA, Menchen P, Cos E, Lorenzo P (2004) Activity of inducible and neuronal nitric oxide synthases in colonic mucosa predicts progression of ulcerative colitis. Am J Gastroenterol 99(9):1756–1764. doi:10.1111/j.1572-0241.2004.40065.x
Singer II, Kawka DW, Schloemann S, Tessner T, Riehl T, Stenson WF (1998) Cyclooxygenase 2 is induced in colonic epithelial cells in inflammatory bowel disease. Gastroenterology 115(2):297–306
Wallace JL (2006) COX-2: a pivotal enzyme in mucosal protection and resolution of inflammation. ScientificWorldJournal 6:577–588. doi:10.1100/tsw.2006.122
Tsubouchi R, Hayashi S, Aoi Y, Nishio H, Terashima S, Kato S, Takeuchi K (2006) Healing impairment effect of cyclooxygenase inhibitors on dextran sulfate sodium-induced colitis in rats. Digestion 74(2):91–100. doi:10.1159/000097657
Hendel J, Nielsen OH (1997) Expression of cyclooxygenase-2 mRNA in active inflammatory bowel disease. Am J Gastroenterol 92(7):1170–1173
Tracey KJ, Cerami A (1994) Tumor necrosis factor: a pleiotropic cytokine and therapeutic target. Annu Rev Med 45:491–503. doi:10.1146/annurev.med.45.1.491
Mitsuyama K, Sata M, Tanikawa K (1991) Significance of interleukin-6 in patients with inflammatory bowel disease. Gastroenterol Jpn 26(1):20–28
Nilsen EM, Johansen FE, Jahnsen FL, Lundin KE, Scholz T, Brandtzaeg P, Haraldsen G (1998) Cytokine profiles of cultured microvascular endothelial cells from the human intestine. Gut 42(5):635–642
Bobin-Dubigeon C, Collin X, Grimaud N, Robert JM, Le Baut G, Petit JY (2001) Effects of tumour necrosis factor-alpha synthesis inhibitors on rat trinitrobenzene sulphonic acid-induced chronic colitis. Eur J Pharmacol 431(1):103–110
Mitsuyama K, Sasaki E, Toyonaga A, Ikeda H, Tsuruta O, Irie A, Arima N, Oriishi T, Harada K, Fujisaki K et al (1991) Colonic mucosal interleukin-6 in inflammatory bowel disease. Digestion 50(2):104–111
Rennick DM, Fort MM (2000) Lessons from genetically engineered animal models. XII. IL-10-deficient (IL)-10(−/−) mice and intestinal inflammation. Am J Physiol Gastrointest Liver Physiol 278(6):G829–G833
Asseman C, Read S, Powrie F (2003) Colitogenic Th1 cells are present in the antigen-experienced T cell pool in normal mice: control by CD4+ regulatory T cells and IL-10. J Immunol 171(2):971–978
Takahashi I, Matsuda J, Gapin L, DeWinter H, Kai Y, Tamagawa H, Kronenberg M, Kiyono H (2002) Colitis-related public T cells are selected in the colonic lamina propria of IL-10-deficient mice. Clin Immunol 102(3):237–248. doi:10.1006/clim.2001.5166
Sydora BC, Tavernini MM, Wessler A, Jewell LD, Fedorak RN (2003) Lack of interleukin-10 leads to intestinal inflammation, independent of the time at which luminal microbial colonization occurs. Inflamm Bowel Dis 9(2):87–97
Foligne B, Nutten S, Grangette C, Dennin V, Goudercourt D, Poiret S, Dewulf J, Brassart D, Mercenier A, Pot B (2007) Correlation between in vitro and in vivo immunomodulatory properties of lactic acid bacteria. World J Gastroenterol 13(2):236–243
Bremner P, Heinrich M (2002) Natural products as targeted modulators of the nuclear factor-kappaB pathway. J Pharm Pharmacol 54(4):453–472
Taylor BS, de Vera ME, Ganster RW, Wang Q, Shapiro RA, Morris SM Jr, Billiar TR, Geller DA (1998) Multiple NF-kappaB enhancer elements regulate cytokine induction of the human inducible nitric oxide synthase gene. J Biol Chem 273(24):15148–15156
Garcia D, Delgado R, Ubeira FM, Leiro J (2002) Modulation of rat macrophage function by the Mangifera indica L. extracts Vimang and mangiferin. Int Immunopharmacol 2(6):797–806
Priulla M, Calastretti A, Bruno P, Azzariti A, Paradiso A, Canti G, Nicolin A (2007) Preferential chemosensitization of PTEN-mutated prostate cells by silencing the Akt kinase. Prostate 67(7):782–789. doi:10.1002/pros.20566
Martelli AM, Nyakern M, Tabellini G, Bortul R, Tazzari PL, Evangelisti C, Cocco L (2006) Phosphoinositide 3-kinase/Akt signaling pathway and its therapeutical implications for human acute myeloid leukemia. Leukemia 20(6):911–928. doi:10.1038/sj.leu.2404245
Tang JM, He QY, Guo RX, Chang XJ (2006) Phosphorylated Akt overexpression and loss of PTEN expression in non-small cell lung cancer confers poor prognosis. Lung Cancer 51(2):181–191. doi:10.1016/j.lungcan.2005.10.003
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Dai, C., Zheng, CQ., Meng, Fj. et al. VSL#3 probiotics exerts the anti-inflammatory activity via PI3k/Akt and NF-κB pathway in rat model of DSS-induced colitis. Mol Cell Biochem 374, 1–11 (2013). https://doi.org/10.1007/s11010-012-1488-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-012-1488-3