Abstract
The Wnt/β-catenin pathway has been proposed as promoting intestinal stem cell division. Wnt ligands activate cytoplasmic β-catenin and increase nuclear translocation of β-catenin that binds to the Tcf-4 transcription factor. The aim of this study was to investigate β-catenin expression in the stem cell region of crypts during intestinal growth in rats. Litters of DAxPVG/c rats were humanely killed at 7, 14, 21, 35, and 72 days of life. β-Catenin and Tcf-4 were quantified by immunoperoxidase staining and image analysis with cumulative signal analysis. Cytoplasmic and nuclear expression of β-catenin peaked nearly 2-fold at day 14 (versus day 7) of life in the stem cell region of intestinal crypts. Tcf-4 nuclear expression peaked earlier at 7 days and was lower thereafter with age. We conclude that the Wnt/β-catenin pathway is activated in the stem cell region of intestinal crypts during growth of the small intestine.
Similar content being viewed by others
Introduction
Factors that influence the postnatal epithelial growth of the small intestine have been investigated since the 1950s [1–4]. It has been difficult to identify a single physiologic growth factor–signaling pathway [3, 4]. Recently, however, the Wnt/β-catenin pathway has been implicated in inducing intestinal stem cell division that results in crypt fission [5–8]. Intestinal stem cells are located at 4 to 5 cell positions from the base of the crypt just above a layer of Paneth cells, although stem cells may admix with Paneth cells in the crypt base [9–12]. It has been proposed that after a threshold of stem cell division, the base of the crypt indents and unzips longitudinally in a process termed crypt fission (or branching) that duplicates crypts [9]. Studies have shown that crypt fission peaks during infancy in mice and rats [13, 14]. Thus, the Wnt/β-catenin pathway should be maximally activated during infancy. However, the changes in β-catenin activation with age in crypts of the small intestine have not been previously investigated.
The Wnt/β-catenin pathway has 3 pathways—namely, the canonical, noncanonical, and calcium signaling pathways. The canonical pathway is the best characterized and the one that is involved in stem cell biology. In man at least, there are 19 Wnt ligands that engage ≥1 of 10 frizzled proteins that are receptors for Wnt ligands. In the unactivated state, β-catenin is anchored in the cell membranes by E-cadherin and is degraded in the cytoplasm by phosphorylation by a multiprotein destructive complex that includes adenomatous polyposis coli, glycogen synthase kinase-3β (GSK3β), and axin/conductin components [15, 16]. Degradation occurs via the ubiquitin proteasome pathway. Wnt ligands bind to the frizzled protein and the low-density lipoprotein related protein 5 and 6 coreceptor and activate disheveled protein that inhibits phosphorylation of GSK3β and thereby allows cytoplasmic accumulation and subsequent nuclear translocation of β-catenin. Activated nuclear β-catenin binds to the Tcf-4 transcription factor. Thus, cytoplasmic and nuclear expression of β-catenin is the hallmark of activation of the β-catenin signaling pathway.
The purpose of this study was to investigate physiologic activation of β-catenin in the stem region of intestinal crypts in infant rats by examining cytoplasmic and nuclear expression of β-catenin. We also examined changes in Tcf-4 expression. Our hypothesis was that activation of β-catenin should peak during infancy when intestinal crypt fission and presumably stem cell division are high.
Methods
Animals
Litters of DAxPVG/c rats were humanely killed at 7, 14, 21, 35, and 72 days of life. Segments of small intestine were removed at approximately one third of the length from the pylorus in the jejunum. The day of birth was designated as day 0. Weaning in the rat occurs from day 14 to 28 of life [17]. Litters were selected with approximately 6–8 pups per litter to equalize nutrition. Each age group had equal members from 2 litters, except for day 35 which consisted of 1 litter of 6 animals. Rat pups were allowed to wean naturally and were only separated from the dam rat after day 28 of life.
Immunostaining
Histologic sections were cut at 4 μm, dewaxed, and rehydrated through ethanol to water. Endogenous peroxidase activity was blocked by incubating with 3% vol/vol hydrogen peroxide in methanol for 1 minute. Immunostaining was performed using an indirect streptavidin–biotin immunoperoxidase technique. Nonspecific binding was blocked by incubating sections with 10% horse or rabbit (i.e., serum from species of secondary antibody) serum in Tris-buffered saline (TBS, pH 7.4) for 20 minutes. β-Catenin was detected by a goat anti–β-catenin antibody (SC-1496, Santa Cruz Biotechnology, Santa Cruz, CA) and Tcf-4 detected by a rabbit anti–Tcf-4 antibody (SC-13027, Santa Cruz Biotechnology). These were diluted at previously determined optimal dilution in 0.05 mol Tris-buffered saline (pH 7.4) containing 2% horse or rabbit (i.e., species of the second antibody) serum and sections incubated overnight at 4°C. A control section was incubated with similarly diluted nonspecific IgG immunoglobulin of the same species as the primary antibody (i.e., goat or rabbit). Bound antibody was detected using level 2 USA Ultra Streptavidin Detection kit (Signet Laboratories, Dedham, MA) except that the secondary antibody was substituted by biotinylated horse anti-goat or anti-rabbit IgG (diluted 1:200 in TBS with 2% horse serum, Vector Laboratories, Burlingham, CA). Color was detected by incubating with diaminobenzidine/0.03% hydrogen peroxide chromogen for 5 minutes.
Quantitation of immunostaining by cumulative signal analysis
Cumulative signal analysis used a Nikon 800 Research microscope (Nikon, Kanagawa, Japan), Spot RT digital camera (Diagnostic Instruments, Sterling Heights, MI) and Image-Pro Plus (Media Cybernetics, Silver Spring, MD), and Matlab software (The Mathworks, Natick, MA) [20–22]. Histologic serial sections were mounted on the same slide so that one section was stained with nonspecific immunoglobulin (control) and the other was stained with specific antibody (experimental). Identical intestinal crypts were located in both control and experimental sections for digital analysis. At 600 magnification (×60 dry lens) an area of interest was located over the cytoplasm or over the nucleus of an enterocyte at 4 or 5 cell positions from the crypt base in the stem cell region. Image matrices were obtained from the TIFF images. The difference of the control and experimental image matrices was obtained and normalized using matrix functions in Matlab and expressed as pixel energy in zero dimension units [18–20]. The average was calculated from 7–13 measurements of crypt cells for each animal. The exact number of animals analyzed depended on the technical quality and orientation of the tissue for microscopic analysis.
Ethics
This study was approved by the Animal Ethics Committee of The Queen Elizabeth Hospital and was carried out according to the Code of Practice for Use of Animals in Research and Teaching of the National Health and Medical Research Council of Australia.
Statistics
Group means were compared using Peritz’ multiple comparison F test [21].
Results
β-catenin cytoplasmic and nuclear localization and quantification of immunostaining
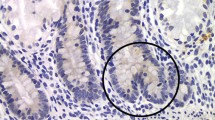
β-Catenin expression was predominantly membrane bound in 7-day-old rats, increased with cytoplasmic and nuclear expression at day 14 of life, decreased in 21-day-old rats, and was only weakly present in 35- and 72-day-old rats (Fig. 1). Cytoplasmic β-catenin was quantified by cumulative signal analysis and peaked 1.8-fold in 14-day-old rats (Fig. 2). Nuclear β-catenin expression was also quantified by cumulative signal analysis and yielded mean values±SE pixel energy values of 47.3±4.9 (n=10), 64.3±5.7 (n=10), and 33.2±4.7 (n=10), respectively, in 7-, 14-, and 21-day-old rats (day 14 versus day 21, P=.0008, Peritz’ F test). Thus, both cytoplasmic and nuclear β-catenin expression had nearly a 2-fold peak at day 14 of life, which indicated activation of the β-catenin pathway.
Comparison of β-catenin staining in intestinal crypts of rats at 7 (a, b), 14 (c, d), and 21 (e, f) days of life. Histologic sections were either stained with nonspecific immunoglobulin as control (a, c, e) or with specific anti–β-catenin antibody (b, d, f). β-Catenin expression was predominantly membrane bound in 7-day-old rats (b), cytoplasmic and nuclear β-catenin expression increased in 14-day-old rats, (d) and low in 21-day-old rats (f). Arrows (d) point to nuclear staining in stem cells of a bifid crypt at day 14 of life. Original magnification ×600
Cytoplasmic expression of β-catenin in the stem cell region of intestinal crypts was measured by immunostaining and cumulative signal analysis in rats from 7–72 days of life. Each age group had 6–11 rats. Data are given as mean values±SE. Day 7 versus day 14, P<.002; day 14 versus day 21, P=.006; day 14 versus day 72, P<.0001; Peritz’ F test
Tcf-4 expression and quantification of nuclear staining
Tcf-4 expression peaked at 7 days of life and was lower thereafter (Fig. 3). Weak staining was present in intestinal crypts and modest staining was present in villous cells. Cytoplasmic staining was present as well as some nuclear staining especially at day 7 of life. The intensity of this staining decreased at day 14 and was weak in 72-day-old animals.
Nuclear expression of Tcf-4 in the stem cell region of intestinal crypts was measured by immunostaining and cumulative signal analysis in rats from 7–72 days of life. Each age group had 5–10 animals. Data are given as mean values±SE. Day 7 versus day 14, P=.025; day 7 versus day 21, P=.0085; day 7 versus day 72, P=.0036; Peritz’ F test
Discussion
We have shown that expression of cytoplasmic and nuclear expression of β-catenin increased nearly 2-fold in the stem cell region of intestinal crypts in 14-day-old rats before declining again. We measured the expression of β-catenin by immunostaining with cumulative signal analysis. The advantage of this method is that it both localizes and quantifies the β-catenin expression to the stem cell region and strongly suggests that the β-catenin pathway is activated in stem cells. It was also evident qualitatively that cytoplasmic and nuclear expression of β-catenin peaked in 14-day-old rats (Fig. 1). Increased cytoplasmic β-catenin expression indicates stabilized activated β-catenin that would come from Wnt signaling, as only this inactivates the destructive APC/GSK3β/axin complex, allowing β-catenin to accumulate cytoplasmically. As the histologic method uses 4-μm sections that slice through the nucleus, any nuclear expression must originate from nuclear translocated β-catenin; there is no cytoplasm in that area of the section. This is further evidence of activated β-catenin that has translocated into the nucleus.
Studies in developmental biology have used genetically manipulated mice with loss of function to show disturbed crypt development or gain of function that show induction of aberrant crypt fission with polyposis [6–8]. The latter is used to infer that the Wnt/β-catenin pathway is important physiologically [6]. Taken together, these studies have demonstrated β-catenin qualitatively in genetically manipulated mice but not quantitatively in the normal animal. Our study directly investigated activation of β-catenin in the normal animal.
Our data also showed an early peak of Tcf-4 expression at day 7 and then decreased levels thereafter with age. Thus, Tcf-4 protein was already present before and during the induction of activated β-catenin at day 14 of life. Our findings are similar to those of Wong et al. [22], who found that Tcf-4 expression slowly declined with age in fetal and neonatal small intestine of the mouse. The early Tcf-4 expression would ensure that the 2 components (i.e., Tcf-4 and β-catenin) of the transcription complex were present to allow transcription of relevant downstream target genes for intestinal growth and division of stem cells.
Wnt ligands could be expressed endogenously in the infant small intestine or be present in breast milk. Limited information is available on distribution of Wnt ligands and frizzled proteins in the mammary gland and the gastrointestinal tract. A study of lactating mammary tissue in mice found that only Wnt6 was still expressed, and Wnt1–5 and -7 were downregulated during lactation [23]. At the time only a limited number of Wnt1-7 ligands were recognized. Pinto et al. [7] allude to unpublished data that Wnt4, 6, 11, and 14B are expressed endogenously in murine fetal and adult small intestine. Ouko et al. [24] detected Wnt11 mRNA in mouse intestine but could not detect other Wnt mRNA. They did not specify the age of their animals, which were presumably adult mice. Wnt14 has been renamed Wnt9A. Clevers et al. [25, 26] have shown in fetal and adult mice that Wnt3, Wnt6, and Wnt9B (previously designated Wnt15) ligands and frizzled-4, frizzled-5, frizzled-6, and frizzled-7 were expressed in the small intestine. Specifically, frizzled-5 and frizzled-7 were exclusively expressed in the crypts of the small intestine at least as specific mRNA detected using in situ hybridization. Frizzled-7 has also been found to be strongly expressed on the epithelium of the small intestine in the chicken [27, 28] and may be therefore expressed in mammalian intestine. Taken together, possible intestinal Wnt ligands are Wnt3, 4, 6, 9B, 11, and 14B (9A), and Wnt6 could be present in breast milk. Possible frizzled proteins are frizzled-4, frizzled-5, frizzled-6, and frizzled-7. These need to be investigated and verified.
In summary, we have found that β-catenin is activated in infant rats with a 2-fold increase in cytoplasmic and nuclear expression (i.e., the hallmark of β-catenin activation) in the stem cell region of intestinal crypts. This supports the notion that the Wnt/β-catenin signaling pathway is a physiologic growth factor for the small intestine.
References
Grand RJ, Watkins JB, Torti FM (1976) Development of the human gastrointestinal tract. A review. Gastroenterology 70:790–810
Montgomery RK, Mulberg AE, Grand RJ (1999) Development of the human gastrointestinal tract: Twenty years of progress. Gastroenterology 116:702–731
Thompson FM, Cummins AG (2000) Growth and maintenance of the small intestinal mucosa. In: Ratnaike R (ed) Small bowel disorders. Edward Arnold Publishing Ltd, London, pp 44–58
Cummins AG, Thompson FM (2002) Effect of breast milk and weaning on epithelial growth of the small intestine in humans. Gut 51:748–754
Korinek V, Barker N, Moerer P, van Donselaar E, Huls G, Petes PJ, Clevers H (1998) Depletion of epithelial stem-cell compartments in the small intestine of mice lacking Tcf-4. Nat Genet 19:379–383
He XC, Zhang J, Tong W-G Tawik O, Ross J, Scoville DH, Tian Q, Zeng X, He X, Wiedemann LM, Mishina Y, Li L (2004) BMP signaling inhibits intestinal stem cell self-renewal through suppression of Wnt-β-catenin signaling. Nat Genet 36:1117–1121
Pinto D, Gregorieff A, Begthel H, Clevers H (2003) Canonical Wnt signals are essential for homeostasis of the intestinal epithelium. Genes Dev 17:1709–1713
Kuhnert F, Davis CR, Wang HT, Chu P, Lee M, Yuan J, Nusse R, Kuo CJ (2004) Essential requirement for Wnt signaling in proliferation of adult small intestine and colon revealed by adenoviral expression of Dickkopf-1. Proc Natl Acad Sci USA 101:266–271
Loeffler M, Birke A, Paulus U, Li Y-Q, Potten CS (1997) Clonality and life cycles of intestinal crypts explained by state-dependent stochastic model of epithelial stem cell organization. J Theor Biol 186:41–54
Wright NA (2000) Epithelial stem cell repertoire in the gut: clues to the origin of cell lineages, proliferation units and cancer. Int J Exp Pathol 81:117–143
Potten CS, Owen G, Booth D (2002) Intestinal stem cells protect their genome by selective segregation of template DNA strands. J Cell Sci 115:2381–2388
Leedham SJ, Brittan M, McDonald SAC, Wright NA (2005) Intestinal stem cells. J Cell Mol Med 9:11–24
Cheng H, Bjerknes M (1985) Whole population cell kinetics and postnatal development of the mouse intestinal epithelium. Anat Rec 211:420–426
Cummins AG, Jones JJ, Thompson FM (2006) Postnatal epithelial growth of the small intestine in the rat occurs by both crypt fission and crypt hyperplasia. Dig Dis Sci 51:718–723
Logan CY, Nusse R (2004) The Wnt signaling pathway in development and disease. Annu Rev Cell Dev Biol 20:781–810
Reya T, Clevers H (2005) Wnt signaling in stem cells and cancer. Nature 434:843–850
Babicky A, Parizek J, Ostadalova I, Kolar J (1973) Initial food intake and growth of young rats in nests of different sizes. Physiol Bohemolov 22:557–566
Matkowskyji KA, Schonfeld D, Benya RV (2000) Quantitative immunohistochemistry by measuring cumulative signal strength using commercial available software Photoshop and Matlab. J Histochem Cytochem 48:303–311
Matkowskyj KA, Cox R, Jensen RT, Benya RV (2003) Quantitative immunohistochemistry by measuring cumulative signal strength accurately measures receptor number. J Histochem Cytochem 51:205–214
Bowen JM, Gibson RJ, Keefe DM, Cummins AG (2005) Cytotoxic chemotherapy upregulates pro-apoptotic Bax and Bak in the small intestine of rats and humans. Pathology 37:56–62
Harper JF (1994) Pertiz’ F test: BASIC programme of a robust multiple comparison test for statistical comparison for statistical analysis of all differences among group means. Comput Biol Med 14:437–445
Wong MH, Huelsken J, Birchmeiert W, Gordon JL (2002) Selection of multipotent stem cells during morphogenesis of small intestinal crypts of Lieberkühn is perturbed by stimulation of Lef-1/β-catenin signaling. J Biol Chem 277:15843–15850
Weber-Hall SJ, Phippard DJ, Niemeyer CC, Dale TC (1994) Developmental and hormonal regulation of Wnt gene expression in the mouse mammary gland. Differentiation 57:205–214
Ouko L, Ziegler TR, Gu LH, Eisenberg LM, Yang VW (2004) Wnt11 signaling promotes proliferation, transformation, and migration of IEC6 intestinal epithelial cells. J Biol Chem 279:26707–26715
Van ESJH, Jay P, Gregorieff A, Van Gijn ME, Jonkheer S, Hatzis P, Thiele A, van den Born M, Begthel H, Brabletz T, Taketo MM, Clevers H (2005) Wnt signaling induces maturation of Paneth cells in intestinal crypts. Nature Cell Biol 7:381–386
Gregorieff A, Pinto D, Begthel H, Destrée O, Kileman M, Clevers H (2005) Expression patterns of Wnt signaling components in the adult intestine. Gastroenterology 129:626–638
McBride HJ, Fatke B, Fraser SE (2003) Wnt signaling components in the chicken intestinal tract. Dev Biol 256:18–33
Theodosiou NA, Tabin CJ. (2003) Wnt signaling during development of the gastrointestinal tract. Dev Biol 259:258–271
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Camac, K.S., Thompson, F.M. & Cummins, A.G. Activation of β-Catenin in the Stem Cell Region of Crypts During Growth of the Small Intestine in Infant Rats. Dig Dis Sci 52, 1242–1246 (2007). https://doi.org/10.1007/s10620-006-9200-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10620-006-9200-7