Article Text
Abstract
Background Hepatic encephalopathy (HE) is defined as brain dysfunction that occurs because of acute liver failure or liver cirrhosis and is associated with significant morbidity and mortality. Lactulose is the standard of care till this date; however, polyethylene glycol (PEG) has gained the attention of multiple investigators.
Methods We screened five databases namely PubMed, Scopus, Web of Science, Cochrane Library and Embase from inception to 10 February 2021. Dichotomous and continuous data were analysed using the Mantel-Haenszel and inverse variance methods, respectively, which yielded a meta-analysis comparing PEG versus lactulose in the treatment of HE.
Results Four trials with 229 patients were included. Compared with lactulose, the pooled effect size demonstrated a significantly lower average HE Scoring Algorithm (HESA) Score at 24 hours (Mean difference (MD)=−0.68, 95% CI (−1.05 to –0.31), p<0.001), a higher proportion of patients with reduction of HESA Score by ≥1 grade at 24 hours (risk ratio (RR)=1.40, 95% CI (1.17 to 1.67), p<0.001), a higher proportion of patients with a HESA Score of grade 0 at 24 hours (RR=4.33, 95% CI (2.27 to 8.28), p<0.0010) and a shorter time to resolution of HE group (MD=−1.45, 95% CI (−1.72 to –1.18), p<0.001) in favour of patients treated with PEG.
Conclusion PEG leads to a higher drop in the HESA Score and thus leads to a faster resolution of HE compared with lactulose.
- hepatic encephalopathy
- lactulose
- cirrhosis
Data availability statement
All data relevant to the study are included in the article or uploaded as supplementary information.
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.
Statistics from Altmetric.com
Introduction
Advanced liver disease is notorious for its systemic complications, particularly its overwhelming effect on brain function. Hepatic encephalopathy (HE), is defined as brain dysfunction that occurs as a result of acute liver failure or liver cirrhosis and is associated with significant morbidity and mortality. It can manifest as a broad spectrum of reversible neuropsychiatric abnormalities, ranging from change in behaviour or personality, intellectual impairment and altered mentation to coma. It is classified as overt HE (OHE) if clinically evident or minimal HE if apparent through neurophysiologic testing.1 2 OHE occurs in 30%–40% of patients with liver cirrhosis and is a frequent cause of hospitalisation; roughly 22 931 hospitalisations occurred secondary to HE in 2009.3 HE is associated with high risk of recurrence, increase in economic burden, poor prognosis and unfortunate quality of life.
One of the main setbacks in early diagnosis and treatment of HE is the lack of a well-validated, gold standard assessment method to detect HE.1 The most widely used HE grading system till this date is the West Haven Criteria (WHC),4 which is a subjective method that divides HE into four stages based on changes in intellectual function, level of consciousness, behaviour and neuromuscular signs.5 Given that it only relies on clinical judgement, it has been heavily criticised for its poor sensitivity in detecting milder forms of HE that are only present with subtle neurocognitive impairments.6 The HE Scoring Algorithm (HESA) is an adaptation of the WHC that possesses both subjective and objective indicators to measure neuropsychiatric symptoms associated with HE (online supplemental table 1) and seems to offer an advantage over other exiting methods as it incorporates a multidimensional approach for the diagnosis of HE.7
Supplemental material
The exact mechanism that leads to HE remains poorly understood. The most accepted theory describes ammonia as a key player in the pathogenesis of HE. In patients with acute liver failure or portosystemic shunts, a buildup of ammonia level in the blood occurs which crosses the blood–brain barrier into the brain and gets metabolised into glutamine by astrocytes. The accumulation of glutamine in the brain increases intracellular osmolarity leading to cerebral oedema.8 Since hyperammonemia has been coined the main contributing factor in the pathogenesis of HE, interventions that target lowering blood ammonia level have been the topic of interest in recent studies.
The initial treatment reported in the literature for the management of HE describes the induction of catharsis with magnesium salts.9 It was not until 1966 when Bircher et al10 published a study regarding the use of lactulose, a non-absorbable disaccharide for the treatment of HE that led to the widespread adoption of this approach. A Cochrane systematic review published by Als-Nielsen et al11 determined that there is no sufficient evidence to support or refute the use of lactulose or other non-absorbable disaccharides for the management of HE. Additional treatment options and preventative interventions for HE are needed to reduce its incidence, alleviate the socioeconomic impact on patients and families and mitigate the burden on healthcare resources.
Polyethylene glycol (PEG) is a safe, affordable, widely available and highly effective osmotic laxative that acts as a faecal cleanser for the removal of faecal nitrogen. Several randomised controlled trials (RCTs) evaluated the effectiveness of PEG versus lactulose in the treatment of HE; however, the results were inconsistent.12–15 Herein, we conducted a systematic review and meta-analysis to assess the safety and clinical efficacy of PEG compared with the standard of care lactulose in patients with HE.
Methods
Study protocol
We followed the steps of the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA)16 during the conduct of this research.
Literature search strategy
We screened five databases—namely PubMed, Scopus, Web of Science, Cochrane Library and Embase—and additional records were identified through other sources such as Google search—from inception to 10 February 2021. We used the following search strategy in all databases: Hepatic encephalopathy AND (polyethylene glycol OR PEG 3350 OR PEG3350 OR Carbowax OR GoLYTELY OR GlycoLax OR Fortrans OR TriLyte OR Colyte OR Halflytely OR macrogol OR MiraLAX OR MoviPrep) AND (lactulose OR Kristalose OR Enulose OR Generlac). There was neither restriction on publication date nor language. Two authors performed the literature search independently and conflicts were resolved by consensus.
Eligibility criteria
We considered all studies that met with the following criteria for our PICOS (Population/Intervention/Comparison/Outcome/Study type) evidence-based research question: (1) patients: individuals with any grade of HE, (2) intervention: PEG, (3) comparator: lactulose, (4) outcomes: reliable data extraction of at least one of our efficacy or safety endpoints and (5) study design: RCTs. We excluded non-randomised studies, conference abstracts, reviews, unpublished RCTs and trials that combined PEG with lactulose as an intervention group. Of note, all included RCTs were open to patients with HE of any grade.
Screening of results
We exported citations from all databases to EndNote software and omitted duplicates. Then, we screened the citations in two steps. We first screened titles and abstracts, and second examined the full texts of potential citations for final inclusion in meta-analysis. Two authors screened the citations and conflicts were resolved by consultation with a third author.
Risk of bias assessment of the included studies
We assessed the risk of bias of included studies according to Cochrane’s risk of bias tool.17 This tool judges the following seven domains: (1) random sequence generation, (2) allocation concealment, (3) blinding of participants and personnel, (4) blinding of outcome assessment, (5) incomplete outcome data, (6) selective outcome reporting and (7) other potential sources of bias. We scored each domain as unclear, low or high risk. Two authors performed the risk of bias assessment and conflicts were resolved by consultation with a third author.
Data extraction
We extracted baseline characteristics of the included studies, such as first author’s name, country, year of publication, study groups, sample sizes, gender, age, cause of liver cirrhosis and precipitant of HE. With regard to efficacy endpoints, we extracted the following: the average HESA Score at 24 hours post treatment, proportion of patients with reduction of HESA Score by ≥1 grade at 24 hours post treatment, proportion of patients with a HESA Score of grade 0 at 24 hours post treatment, length of hospital stay and time to resolution of HE. With regard to safety endpoints, we extracted the following: frequency of patients with hypokalemia and frequency of death. HESA Score is a widely accepted instrument to grade the severity of HE.7 Resolution of HE was defined as the reduction of at least one grade of HESA Score at 24 hours post treatment. Hypokalemia was defined as concentration level less than 5 μmol/L at 24 hours post treatment.
Data analysis
We used Review Manager software V.5.4.0 for statistical analysis. We analysed dichotomous and continuous data using the Mantel-Haenszel and inverse variance methods, respectively. We pooled the outcomes as risk ratios (RRs) or weighted mean differences, respectively, with 95% CIs. We considered the fixed and random effects models for homogeneous and heterogeneous pooled outcomes, respectively. Between-study heterogeneity was defined as χ2 p<0.1 and I2 test>50%.18 If applicable, we performed a sensitivity analysis to rectify the heterogeneous pooled outcomes. During sensitivity analysis, we would eliminate one RCT at a time and recalculate the summary RRs for the remaining RCTs. We did not assess for publication bias using Egger’s funnel plots, because of the small number of included studies.19
Results
Literature search
Figure 1 displays the PRISMA diagram. Our literature search yielded a total of 148 citations, of which 64 citations were identified as duplicates. Afterward, we screened the titles and abstracts of the remaining 84 citations and only 6 citations were advanced to full-text screening. Finally, only four RCTs met our inclusion criteria and were included in the qualitative and quantitative analysis.12–15 The baseline characteristics of the included studies are depicted in table 1. Overall, there were a total of 229 patients (121 and 108 patients received PEG and lactulose, respectively).
Baseline characteristics of the included studies
Preferred Reporting Items for Systematic Reviews and Meta-Analysis flowchart.
Risk of bias assessment
Overall, all studies, except one,20 provided adequate details regarding random sequence generation and allocation concealment. Moreover, all studies were designed as open label and we scored the performance bias domain as high risk. Lastly, two studies did not provide accessible preregistered study protocol and we scored the selection bias domain as high risk. Figure 2 depicts the risk of bias summary and graph of all included studies.
Risk of bias graph and summary of the include studies.
Efficacy endpoint: average HESA Score at 24 hours post treatment
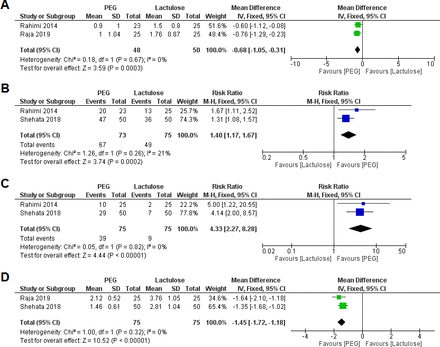
Two RCTs were analysed, comprising a total of 98 patients (PEG=48, lactulose=50). The pooled effect size demonstrated a significantly lower average HESA Score at 24 hours post treatment in favour of the PEG group (MD=−0.68, 95% CI (−1.05 to –0.31), p<0.001). The pooled results were homogeneous (I2=0%, p=0.67) (figure 3A).
Forest plot for meta-analysis of average HESA Score at 24 hours post treatment (A), proportion of patients with reduction of HESA Score by ≥1 grade at 24 hours post treatment (B), proportion of patients with a HESA Score of grade 0 at 24 hours post treatment (C) and time to resolution of HE (D). HESA, Hepatic Encephalopathy Scoring Algorithm; PEG, polyethylene glycol.
Efficacy endpoint: proportion of patients with reduction of HESA Score by ≥1 grade at 24 hours post treatment
Two RCTs were analysed, comprising a total of 148 patients (PEG=73, lactulose=75). The pooled effect size demonstrated a significantly higher proportion of patients with reduction of HESA Score by ≥1 grade at 24 hours post treatment in favour of the PEG group (RR=1.40, 95% CI (1.17 to 1.67), p<0.001). The pooled results were homogeneous (I2=21%, p=0.26) (figure 3B).
Efficacy endpoint: proportion of patients with a HESA Score of grade 0 at 24 hours post treatment
Two RCTs were analysed, comprising a total of 150 patients (PEG=75, lactulose=75). The pooled effect size demonstrated a significantly higher proportion of patients with a HESA Score of grade 0 at 24 hours post treatment in favour of the PEG group (RR=4.33, 95% CI (2.27 to 8.28), p<0.001). The pooled results were homogeneous (I2=0%, p=0.82) (figure 3C).
Efficacy endpoint: time to resolution of HE
Two RCTs were analysed, comprising a total of 150 patients (PEG=75, lactulose=75). The pooled effect size demonstrated a significantly shorter time to resolution of HE in favour of the PEG group (MD=−1.45, 95% CI (−1.72 to –1.18), p<0.001). The pooled results were homogeneous (I2=0%, p=0.32) (figure 3D). Rahimi et al reported that the median time to resolution of HE was significantly shorter in favour of the PEG group compared with lactulose group (1 day vs 2 days, p=0.01, respectively).
Efficacy endpoint: length of hospital stay
Four RCTs were analysed, comprising a total of 294 patients (PEG=147, lactulose=147). The pooled effect size did not demonstrate a significant difference in terms of length of hospital stay between both groups (MD=−1.00, 95% CI (−1.99 to –0.01), p=0.05). The pooled results were heterogeneous (I2=78%, p=0.003) (figure 4A). Between-study heterogeneity was best resolved on the omission of the study by Shehata et al13 and the repooled effect size failed to demonstrate a significant difference between both groups (MD=−0.55, 95% CI (−1.54 to 0.44), p=0.27; heterogeneity: I2=50%, p=0.14) (figure 4B).
Forest plot for meta-analysis of length of hospital stay before (A) and after (B) sensitivity analysis. PEG, polyethylene glycol.
Safety endpoint: frequency of patients with hypokalemia at 24 hours post treatment
Two RCTs were analysed, comprising a total of 144 patients (PEG=72, lactulose=72). The pooled effect size did not demonstrate a significant difference between both groups with regard to the frequency of patients with hypokalemia at 24 hours post treatment (RR=0.90, 95% CI (0.40 to 2.04), p=0.80). The pooled results were homogeneous (I2=0%, p=0.75) (figure 5A).
Forest plot for meta-analysis of safety endpoints namely frequency of patients with hypokalemia at 24 hours post treatment (A) and frequency of death (B). M-H, Mantel-Haenszel; PEG, polyethylene glycol.
Safety endpoints: frequency of death
Two RCTs were analysed, comprising a total of 150 patients (PEG=100, lactulose=100). The pooled effect size did not demonstrate a significant difference between both groups with regard to the frequency of death (RR=0.50, 95% CI (0.13 to 1.94), p=0.32). The pooled results were homogeneous (I2=0%, p=1.00) (figure 5B).
Discussion
Despite improved knowledge on the pathophysiology of HE, the therapeutic options available for HE has experienced only mild changes with non-absorbable disaccharides being the first line of treatment. PEG is an inexpensive, safe and widely used medication for the treatment of constipation and its use in HE has recently caught the attention of multiple investigators. This meta-analysis is the first one in the literature to compare the effect of PEG versus lactulose in the management of HE. Our analysis has showed that the use of PEG compared with lactulose in patients with HE resulted in a significantly lower average HESA Score at 24 hours post treatment in favour of the PEG group. Additionally, a significantly higher proportion of patients in the PEG group had a reduction of HESA Score by ≥1 grade at 24 hours post treatment compared with lactulose and a significantly higher proportion of patients in the PEG group had HESA Score of grade 0 at 24 hours. In fact, two studies by Naderian et al21 and Ahmed et al22 compared the concomitant use of PEG with lactulose versus lactulose and concluded that the combination had led to a higher 24 hours HESA Score change as well as a higher reduction in HESA Score by more than one grade at 24 hours post treatment.
The mechanism by which PEG improved HESA Score remains debatable. It has been revealed that lactulose works by reducing intestinal ammonia production and absorption leading to an improvement in HE;23 however, when comparing ammonia levels 24 hours after administration of PEG or lactulose, two trials12 15 showed that lactulose was associated with a higher drop in the mean ammonia level compared with patients that were in the PEG group, suggesting that improvement in symptoms of HE in patients that were given PEG was not entirely due to clearance of ammonia. It is important to note that the serum ammonia level does not always correlate with the severity of clinical symptoms, and clinical analysis has showed that lactulose was related to a non-response rate as high as 22%.24
HE-related hospitalisation costs continue to rise; these costs escalated from $4.68 billion in 2005 to $7.25 billion in 2009.3 Our analysis showed that patients treated with PEG had a significantly shorter time to resolution of HE. Naderian et al21 found that patients treated with PEG and lactulose had a shorter mean hospital stay of 6.8 days compared with patients treated with lactulose alone (mean 8.9 days). These results were also validated by Ahmed et al22 who found that patients treated with both PEG and lactulose had a mean hospital stay of 9 days compared with 13 days in patients treated with lactulose alone. However, despite the fact that the studies included in this analyses8–11 commented on the shorter length of hospital stay in patient receiving PEG, our analysis failed to show statistical significance in terms of length of hospital stay even after omitting one study to create a more homogenous group. Accordingly, while the use of PEG contributed to a faster reduction in HESA Score and faster resolution of HE, however, it failed to show a statistical significance in terms of shorter length of hospital stay.
An important consideration with the use of PEG is that it causes substantial catharsis and in theory may lead to dehydration and electrolytes instabilities. However, our study has showed that there was no difference between both groups with regard to the frequency of patients with hypokalemia at 24 hours post treatment. The use of PEG in HE was considered safe and tolerable and was not associated with major serious adverse events. The adverse events reported were gastrointestinal in nature such as diarrhoea, nausea and abdominal distension.12 13 In three of the studies, one patient in each study receiving PEG died, however, the cause of death was not related to the intervention itself and our study showed that there was no significant difference between both groups with regard to the frequency of death.
Some limitations still existed in our meta-analysis. First, we included a small number of studies with a small number of patients. When comparing clinical improvement with changes in the HESA, the forest plots only compared two studies and therefore the numbers were low. We also did not include trials comparing the combination of PEG with lactulose versus lactulose as the aim of the study was to compare the two interventions ‘head’ to ‘head’. Larger RCTs are still needed to validate the results. Second, there was some clinical heterogeneity in our study. This may be attributed to one trial12 where its protocol stipulated that potential participants could be treated with a single dose of lactulose prior to randomisation, thus patients in the PEG group might have received lactulose before being assigned to the PEG group. Also, in consideration of small number of RCTs per outcome, the degree of between-study homogeneity should be interpreted with caution.25 Third, although our analysis showed that patients in the PEG group had faster resolution of HE, it is important to note that for ethical considerations, the trials were designed to avoid delay of standard of care for more than 24 hours at which point patients received lactulose. Whether the faster resolution of HE is due to PEG alone or from the combination of both PEG and lactulose is unknown. Fourth, since the number of included studies was low (less than 10 RCTs), we did not perform publication bias analysis and hence our results could be liable to bias in the reported outcomes.
In conclusion, compared with the standard of care lactulose, a single dose of PEG significantly improved the 24 hours HESA Score and reduced the number of days for HE resolution. Since PEG is safe, widely used and easy to administer, the use of PEG should be considered in the treatment of HE, however further studies are required to validate these result and better understand its effect on encephalopathy-related quality of life.
Data availability statement
All data relevant to the study are included in the article or uploaded as supplementary information.
References
Supplementary materials
Supplementary Data
This web only file has been produced by the BMJ Publishing Group from an electronic file supplied by the author(s) and has not been edited for content.
Footnotes
Contributors GJH: study conception and design, acquisition of data, statistical analysis, interpretation of data, drafting of the manuscript, critical revision of the manuscript for important intellectual content and submission of the manuscript. MFA: drafting of the manuscript and critical revision of the manuscript for important intellectual content. JNH: data analysis, interpretation of data, drafting of the manuscript and critical revision of the manuscript for important intellectual content. AA-Z: acquisition of data, interpretation of data, statistical analysis, drafting of the manuscript and critical revision of the manuscript for important intellectual content. CD and SD: acquisition of data and drafting of the manuscript. TA: critical revision of the manuscript for important intellectual content. SJ: critical revision of the manuscript for important intellectual content, statistical analysis and study supervision and is also responsible for the overall work as a guarantor.
Funding The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests None declared.
Provenance and peer review Not commissioned; externally peer reviewed.
Supplemental material This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.