Article Text
Abstract
Introduction The medical treatment options for patients with Crohn’s disease (CD) are limited and patients resistant to those therapies are left requiring surgical operations that usually only achieve some symptomatic relief. Mesenchymal stem cells (MSC) have been shown to be effective for the treatment of CD, and we have demonstrated in animal experiments that human amnion-derived MSCs (AMSC) are a potential new therapeutic strategy. Therefore, we designed this study to investigate the safety and efficacy of AMSCs in patients with treatment-resistant CD.
Methods and analysis This is the protocol for an ongoing phase I/II, dual-centre, open-label, uncontrolled, dose–response study. The estimated enrolment is 6–12 patients with treatment-resistant, moderate CD. A dose of 1.0×106 cells/kg will be administered intravenously in the low-dose group at days 0 and 7. After confirming the safety of low-dose administration, a dose of 4.0×106 cells/kg will be administered intravenously in the high-dose group on days 0 and 7. The primary endpoint will measure the occurrence of adverse events related to acute infusion toxicity, and secondary endpoints will include long-term adverse events and efficacy of AMSC administration.
Ethics and dissemination The Institutional Review Board of Hokkaido University Hospital approved this study protocol (approval number H29-6). A report releasing study results will be submitted to an appropriate journal.
Discussion This study is the first to investigate the safety and efficacy of AMSC use for CD treatment. Our results will advance studies on more efficient and convenient methods to overcome the limits of available CD treatments.
Trial registration number UMIN000029841.
- Crohn’s disease
- stem cells
- bacterial interactions
This is an Open Access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Statistics from Altmetric.com
Introduction
Crohn’s disease (CD) is a chronic inflammatory gastrointestinal disease occurring mainly in young people. Inflammation may affect any portion of the digestive system from the mouth to the anus, with characteristic pathological lesions such as intestinal stenosis or fistulas. In addition, complications may develop in other organs such as joints, skin, or eyes. The main symptoms include digestive symptoms such as diarrhoea and abdominal pain, and symptoms derived from complications such as fever, weight loss, and malnutrition. The disease progresses with relapses and remission periods often impairing social life.1 2
Medical treatment for CD includes the administration of 5-amino salicylic acid, steroids, immunomodulators, anti-tumour necrosis factor (TNF) antibodies, anti-interleukin (IL)-12/23p40 antibody, and antibiotics, with the choice of treatment depending on the location and severity of disease.3 4 The development of new therapeutic drugs has increased treatment options, but many patients are resistant to the available medication, and approximately 50% of patients requiring surgical operation for symptomatic relief within 10 years of diagnosis.5 6 New treatment strategies are required for refractory CD resistant to existing therapies.
Mesenchymal stem cells (MSC) are multipotent, plastic adherent stromal cells present in a variety of tissues, including bone marrow, fat, and umbilical cords. MSCs are considered a potential cell source for regenerative medicine because they can secrete a variety of humoral anti-inflammatory factors that are required for tissue regeneration.7 Reports have suggested the efficacy of MSCs for CD treatment.8
We have demonstrated that a large number of MSCs can be obtained from the amnion, which is discarded after delivery, and that intravenous administration of human amnion-derived MSCs (AMSC) alleviates inflammatory bowel disease in dextran sulfate sodium-induced colitis and trinitrobenzene sulfonic acid-induced colitis rat models.9 10 We believe that the anti-inflammatory effects of AMSCs are responsible for those effects.
Therefore, the present study has been designed to investigate the safety and efficacy of intravenous administration of AMSCs (AM01) in patients with treatment-resistant CD, in a phase I/II clinical trial.
Methods and analysis
Study design
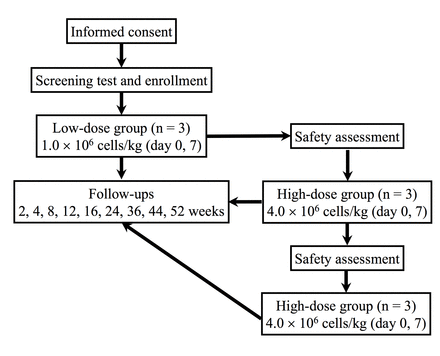
This study is a phase I/II, dual-centre, open-label, uncontrolled, dose–response clinical trial (figure 1), currently under way at the Hokkaido University Hospital in Sapporo, Japan, and in the Hyogo College of Medicine College Hospital in Nishinomiya, Japan. This trial is registered at the University Hospital Medical Information Network Clinical Trial Registry (UMIN000029841). The total study period is 2 years, from November 2017 to October 2019.
Schematic of the clinical trial.
Study population
Inclusion criteria
Men or women aged 20–69 years.
Patients with a definitive diagnosis of CD at least 24 weeks before registration in the study, according to the guidelines established by the Research Group of Intractable Inflammatory Bowel Disease, subsidised by the Ministry of Health, Labor, and Welfare of Japan and the Guidelines Committee of the Japanese Society of Gastroenterology.1
Patients with CD resistant to anti-TNF-α and anti-IL12/23p40 antibody treatment, that is, those who cannot mount an initial clinical response or those who fail to respond further after mounting a primary response, or patients intolerant to the treatment due to their adverse or side effects.
Patients with a Crohn’s Disease Activity Index (CDAI) between 200 and 450.
Patients with a main lesion in the ileum end or the colorectum.
Patients who voluntarily sign the Institutional Review Board-approved written informed consent form.
Exclusion criteria
Patients with fistula and uncontrollable abscess (ie, resistant to antibiotics and requiring drainage or surgery).
Patients with a history of total or subtotal colectomy.
Patients with a history of small intestinal resection or short bowel syndrome.
Patients with stoma, internal fistula, or severe intestinal stricture.
Patients who underwent surgery within 4 weeks before signing the informed consent.
Patients with a history of cancer during the last 5 years.
Patients who received any one of the medicines or treatments not allowed before registration such as cytoapheresis, cyclosporine, tacrolimus, antibiotics, total parenteral nutrition, total enteral nutrition, intravenous administration of steroid, >30 mg/day of oral steroid, laxative, enema, colectomy, live attenuated vaccine, and other clinical trial medicines or treatments; during the trial, patients may receive medicines or treatments such as 5-aminosalicylic acid (5-ASA), ≤30 mg/day of oral steroid, antidiarrhetic medication, antiflatulent agents, probiotics, prebiotics, infliximab, adalimumab, ustekinumab, azathioprine, 6-mercaptopurine (6-MP), methotrexate (MTX), elemental diet, and oligomeric formula. However, during the study, participants are not allowed to start, stop, or change the dosages of those treatments. In addition, the doses of 5-ASA, steroids, antidiarrhetics, diarrhoea-predominant irritable bowel syndrome, antiflatulents, probiotics, and prebiotics must not have been modified during the 2 weeks prior to enrolment. And, the doses of infliximab, adalimumab, azathioprine, 6-MP, MTX, elemental diet, and oligomeric formula must not have been modified during the 8 weeks prior to enrolment (the doses of ustekinumab must not have changed for 16 weeks prior to enrolment).
Patients with uncontrollable systemic disease.
Patients with the following laboratory test result values:
Haemoglobin <8.0 g/dL.
White cell count <3.0x10^9/L.
Lymphocyte count <500 cells/ µL.
Aspartate aminotransferase >3 times the upper limit.
Alanine aminotransferase >3 times the upper limit.
Total bilirubin >2.0 mg/dL.
Serum creatinine >2 times the upper limit.
Patients who cannot keep a room air SpO2 ≥94%.
Patients positive for HBs-Ag, HCV-Ab, HIV-Ab, or HTLV-1-Ab.
Patients with any severe uncontrollable infectious disease.
Patients with severe hypersensitivity to bovine-derived constituents, human serum albumin, or gentamicin.
Patients with cancer, dysplasia, or adenomatous polyps in the colorectum that require treatment.
Patients with a history of hypersensitivity to iodine or iodine-containing contrast agents.
Patients who have undergone any other treatment with cell therapy products.
Patients with a history of any severe neurological disorders.
Pregnant or breastfeeding women, those who want to become pregnant, or those who cannot use contraceptives.
Patients deemed by the investigators as unsuitable for participation in the study.
Screening and implementation
Before enrolment, the investigator assesses the eligibility of potential participants according to the inclusion and exclusion criteria. Screening tests are performed after signing informed consent forms. The detailed screening items to assess in the participants are described below:
Patient’s biographical characteristics
Medical and concomitant drug use histories.
Physical examination and vital signs.
Blood sampling for serological tests, including complete blood count, biochemical tests, HBs-Ag, HCV-Ab, HIV-Ab, HTLV-1-Ab.
Urinalysis including urine hCG-β.
SpO2.
Chest X-ray.
ECG.
CT scan.
Objective outcome measures
CDAI.
Inflammatory Bowel Disease Questionnaire (IBDQ) scores.
Simple Endoscopic Score for CD (SESCD).
AM01 administration should be performed within 1 week of performing the screening tests. All detailed procedures are described in table 1.
The schedule of the time of administration
Sample size
Because this trial is a phase I/II pilot study, each group will comprise three patients to examine the safety of the AM01 injection according to a dose escalation protocol. The sample size of the safety test is based on the traditional 3+3 dose escalation design.11 Safety in the high-dose group will be evaluated after confirmation of safety in the three cases of the low-dose group. After confirmation of safety in the three cases of the high-douse group, additional three cases will be enrolled for the high-dose group to explore the efficacy.
Statistical analysis
A full statistical analysis plan will be written prior to data collection. Objective outcome measures will be scored according to standard methodology, with prior knowledge of the limitations of the small sample size. We will measure CDAI, SESCD, and IBDQ to analyse the effectiveness of AMSCs and prepare figures showing changes in those scores for each patient.
The characteristics of AMSCs
This study uses AMSCs (AM01) supplied by Dr Kenichi Yamahara,12 and approved for clinical studies by the Pharmaceuticals and Medical Devices Agency (PMDA, Tokyo, Japan). The phenotypes of AM01 are characterised by their positivity for CD73, CD90, and CD105, and by their negativity for haematopoietic-associated marker CD45 and epithelial cell-associated marker CD326.
Preparation of AMSCs
After obtaining informed consent, human fetal membranes were obtained during caesarean deliveries, and the amnion was separated from the chorion by peeling. AMSCs were isolated and expanded by digestion using several Good Manufacturing Practice (GMP)-grade enzymes. The cells were then seeded in plastic cell culture chambers with basal medium supplemented with adult bovine-derived platelet-rich plasma (NeoSERA, Japan Biomedical, Otofuke, Japan, https://www.japan-biomedical.jp). The cultures were maintained at 37°C in a humidified atmosphere of 95% air and 5% CO2. Next, the cells were harvested via trypsinisation, and AMSCs were packaged into frozen bags and stored under −130°C. Cells were further cultured after thawing the frozen bags, passaged, harvested, and packaged into frozen bags and stored below −130°C as product doses. Quality testing for AM01 included assessments for cell appearance, purity, viable cell number, viability, and presence of bacteria, virus, mycoplasma or endotoxin contamination. We followed the GMP guidelines for regenerative medicine of the PMDA during the whole manufacturing process.
Intervention
A dose of 1.0×106 cells/kg will be administered intravenously to patients in the low-dose group on days 0 and 7, and a dose of 4.0×106 cells/kg will be administered intravenously to those in the high-dose group on days 0 and 7.
Endpoints of the study
Primary endpoint
Identification of adverse events related to acute infusion toxicity (within 24 hours after infusion).
Secondary endpoints
Identification of adverse events during the 52 weeks following the first administration of AM01.
Identification of defects of investigational products.
Assessment of CDAI, SESCD, and IBDQ results 4 weeks after the first administration of MSCs, and of time-course changes during 52 weeks after the first administration.
Identification of infiltrating macrophages and neutrophils in inflamed tissue.
Measurement of serum levels of proinflammatory markers (TNF-α, MCP-1, IL-1β, MIF) and the anti-inflammatory marker IL-10.
Safety assessment and reporting of adverse events
Patients will be hospitalised from day −1 through day 8. Adverse events and safety assessments will be conducted during AM01 administration, immediately after AM01 administration, 15 min, 30 min, 1 hour, 2 hours, 4 hours, 6 hours, and 24 hours after AM01 administration (table 1), and at every visit after AM01 administration. The investigator will report adverse events and protect patients who participate in this trial. Adverse events and their associations with injected materials will be reported according to the Common Terminology Criteria for Adverse Events v4.0 of the Japan Clinical Oncology Group grading. Severe adverse events that result in life-threatening and prolonged disability conditions will be reported to the institutional review board by the investigator.
Dropping out policy
Participants have the right to discontinue participating in the trial at any time without stating any reason. Moreover, participants may be excluded because of medical or other reasons as follows:
Inability to continue participation because of relocation.
Violation of inclusion criteria.
A situation in which injecting AM01 is deemed impossible due to adverse events.
A situation in which injection of AM01 is deemed impossible due to product quality problems.
Participants who fulfil the exclusion criteria for the second injection.
An investigator may decide to exclude a patient because of health and well-being concerns.
Follow-up of study
The patients will be hospitalised from day −1 through day 8 and will visit the hospital thereafter during the 2nd, 4th, 8th, 12th, 16th, 20th, 24th, 28th, 36th, 44th, and 52nd weeks (table 2). Details of the examinations are summarised as follows:
Physical examination, vital signs, recording, and identification of adverse events: during every visit.
Serological and biochemical test, urinalysis: during every visit.
Pregnancy tests for women: at weeks 4, 12, 24, 36, and 52.
ECG: at every visit.
CT scan: at weeks 24 and 52.
Chest X-ray: at weeks 2, 12, and 36.
CDAI: at every visit.
IBDQ: at weeks 4, 24, and 52.
SESCD: at weeks 4, 24, and 52.
The schedule of the study
Data collection and monitoring
The committee supervises data collection and management for all the procedures during the trial. An appointed researcher approved by the committee collects all the relevant data. Data collections are detailed by Standard Operating Procedures of the Good Clinical Practice and International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use guidelines.
Discussion
Patients with CD with active disease lesions often experience decreased quality of life. Anti-TNF antibodies, especially infliximab, have had a great impact on CD treatment because of their ability to induce and maintain remission. However, about one-third of the prescribed patients do not respond to induction therapy (primary non-response), and 25%–40% of those who initially respond lose the response during treatment (secondary loss of response).13 Switching to another anti-TNF-α antibody (adalimumab) or to the anti-IL12/23p40 antibody (ustekinumab) is an alternative treatment for these patients, but there are no additional possibilities for patients that are refractory to these treatments.
The main mechanisms of MSCs for tissue regeneration and repair are believed to involve differentiation into target cells, paracrine effects by secretion of humoral factors, and immune regulation.14 Among those mechanisms, when MSCs are administered systemically, the latter two are considered to be particularly active.15
Clinical trials using MSCs to treat patients with CD have been conducted; and administration of autologous bone marrow-derived MSC treatment for refractory luminal CD has been reported to be safe and feasible resulting in ameliorated clinical symptoms and endoscopic findings without any critical side effects.16 In addition, administration of allogeneic bone marrow-derived MSCs to patients with biologic therapy-refractory CD improved the CDAI and the Crohn’s Disease Endoscopic Index of Severity.17 Moreover, allogeneic transplantation of MSCs obtained from the bone marrow of healthy donors and from umbilical cords obtained during normal deliveries was safe and may have contributed to the clinical improvement seen in patients with refractory CD and ulcerative colitis18
In this study, we are using AMSCs, obtained from human amnion specimens. AMSCs are promising cells because large amounts can be obtained from an amnion, and amnions can be obtained after delivery without any invasive procedures inflicted upon the donors.
This study has the limitation of its small sample size. Although the study population has been determined by traditional 3+3 dose escalation with modification, we will not be able to draw general conclusions for the effects of AMSCs. However, this study is the first in-human clinical trial to use AMSCs. The results of this pilot study will help to determine a safe dose of AMSCs for clinical use. In addition, our results and experience will help plan for future clinical trials using AMSCs for CD treatment.
In conclusion, our study is the first to investigate the safety and efficacy of AMSCs used for treating CD. We expect that our results will expand knowledge for more efficient and convenient methods to overcome the limits of the available CD treatments.
Dissemination plan
At the end of the study, a report describing the study results will be submitted for publication in an appropriate journal after completion of data analysis.
Acknowledgments
The authors thank Dr Takahiro Yamada and Dr Takafumi Kojima for performing caesarean deliveries.
References
Footnotes
Contributors SOt contributed by writing the manuscript and in carrying out the trial. SOh is the principal investigator, responsible for conception, design, and processing of the trial. AM, HH, IK, and TI reviewed all protocol versions and contributed to the start-up of the trial. YMI contributed to the design of the trial and the statistical analysis plan. TK and SN contributed by reviewing all protocol versions and carrying out the trial. KY reviewed all protocol versions and was involved in manufacturing and quality control of AM01. NSat and NSak supervised and edited the protocol.
Funding This work was supported by the Research Project for Applications of Regenerative Medicine from the Japan Agency of Medical Research and Development (AMED, 17bk0104058h0002).
Competing interests None declared.
Patient consent Not required.
Ethics approval Institutional Review Board of Hokkaido University Hospital (approval number H29-6).
Provenance and peer review Not commissioned; externally peer reviewed.
Data sharing statement We will share the data after the trial is finished. Additional details of the study protocol can be requested from the corresponding author.