Article Text
Abstract
Objectives Bowel cleansing is necessary before colonoscopy, but is a burden to patients because of the long cleansing time and large dose volume. A low-volume (2 L) hypertonic polyethylene glycol-ascorbic acid solution (PEG-Asc) has been introduced, but its possible dehydration effects have not been quantitatively studied. We compared the efficacy and safety including the dehydration risk between hypertonic PEG-Asc and isotonic PEG regimens.
Design This was an observer-blinded randomised study. Participants (n=310) were allocated to receive 1 of 3 regimens on the day of colonoscopy: PEG-Asc (1.5 L) and water (0.75 L) dosed with 1 split (PEG-Asc-S) or 4 splits (PEG-Asc-M), or PEG-electrolyte solution (PEG-ES; 2.25 L) dosed with no split. Dehydration was analysed by measuring haematocrit (Ht).
Results The cleansing time using the hypertonic PEG-Asc-S (3.33±0.48 hours) was significantly longer than that with isotonic PEG-ES (3.05±0.56 hours; p<0.001). PEG-Asc-M (3.00±0.53 hours) did not have this same disadvantage. Successful cleansing was achieved in more than 94% of participants using each of the 3 regimens. The percentage changes in Ht from baseline (before dosing) to the end of dosing with PEG-Asc-S (3.53±3.32%) and PEG-Asc-M (4.11±3.07%) were significantly greater than that with PEG-ES (1.31±3.01%).
Conclusions These 3 lower volume regimens were efficacious and had no serious adverse effects. Even patients cleansed with isotonic PEG-ES showed significant physiological dehydration at the end of dosing. The four-split PEG-Asc-M regimen is recommended because of its shorter cleansing time without causing serious nausea.
Trial registration number UMIN000013103; Results.
- COLONOSCOPY
- ENDOSCOPY
- ENDOSCOPIC PROCEDURES
This is an Open Access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Statistics from Altmetric.com
Summary box
What is already known about this subject?
▸ A hypertonic low-volume polyethylene glycol-ascorbic acid solution (PEG-Asc, 2 L) has been introduced to decrease the volume of isotonic polyethylene glycol-electrolyte solution (PEG-ES, 4 L) that patients are required to drink for bowel preparation before colonoscopy.
▸ Patients either spent 2 days taking the split dose the day before and on the day of procedure, or spent 1 day taking the same-day full dose. The 2 L same-day dose of PEG-Asc causes greater disturbance to the intestinal microbiota than the split dose.
▸ To further decrease patient's burden and the hypertonic effects on the gut, it is worth exploring lower volume same-day regimens of PEG-Asc.
What are the new findings?
▸ Successful cleansing was achieved in more than 94% of participants with the same-day dose of two regimens of 1.5 L of PEG-Asc taken as one-split dosing (intake of 1 and 0.5 L) and as four-split dosing (five intakes of 0.3 L), and one regimen of 2.25 L PEG-ES with no split.
▸ PEG-Asc with four-split dosing had a significantly shorter preparation time (3.00±0.53 hour) compared with PEG-Asc with one-split dosing.
▸ Percentage changes in haematocrit from baseline to the end of dosing with the one-split and the four-split PEG-Asc regimens (3.5% and 4.1%, respectively) were greater than that with PEG-ES (1.3%), although isotonic PEG-ES had previously been assumed to cause no change in physiological dehydration.
How might it impact on clinical practice in the foreseeable future?
▸ A lower dose of 1.5 L PEG-Asc with four-split dosing is a viable new regimen because of its efficacy, safety and shorter cleansing time. Patients who had PEG-Asc were slightly dehydrated at the end of the colonoscopy; hence, additional water intake is recommended.
Introduction
Isotonic polyethylene glycol-electrolyte solutions (PEG-ES) are widely used as bowel cleansing agents before colonoscopy. A 4 L volume of PEG-ES is frequently used but the regimen is a burden to patients because of the long cleansing time and large dose volume. Low-volume regimens which were introduced to reduce the volume that patients must drink are now more frequently used; one of these low-volume regimens is polyethylene glycol-ascorbic acid solution (PEG-Asc; Moviprep).1–10 For example, 2 L of PEG-Asc can be taken either divided as 1 L on the evening before and 1 L on the morning of the procedure (split dosing), or taken as 2 L on the day of the procedure (same-day dosing). It is also recommended that patients drink a further 1 L of clear liquid to prevent the feelings of thirst and dehydration.11
PEG-Asc contains higher amounts of PEG (1.7-fold) compared with PEG-ES and sodium sulfate (1.3-fold), NaCl, KCl, and ascorbic acid and sodium ascorbate. PEG, sodium sulfate and excess ascorbic acid are not absorbable from the gastrointestinal tract,12–14 and exert an osmotic action in the colon that causes increased laxative effects. While the extent of dehydration using hypertonic PEG-Asc has not yet been clarified, it has been suggested that isotonic PEG-ES causes little fluid exchange across the colonic mucosal membrane.15 A recent MRI study demonstrated that the intake of PEG-Asc causes distention of the ascending and transverse colons, stimulating colonic motility and resulting in defecation; this prevents the total colon volume increasing beyond an average of 1.2 L.16 Hence, an intake volume of <2 L of PEG-Asc on the day of colonoscopy would be an attractive regimen if it were still efficacious.
In the present study, we explored the effects of lower volume PEG-Asc same-day regimens using either a four-split dose or a one-split dose on efficacy and safety including dehydration. We used isotonic PEG-ES solution with no-split dose as a control.
Materials and methods
This study was conducted in Akita Red Cross Hospital, Akita, Japan from December 2013 to July 2014. The study protocol was approved by the Institutional Review Board of this hospital, and the study was performed in accordance with the Declaration of Helsinki and Good Clinical Guidelines. Written informed consent was obtained from all patients. This study was registered with the University Hospital Medical Information Network (UMIN) Clinical Trials Registry System http://www.umin.ac.jp/ctr/index.htm (UMIN000013103).
Patients
Eligible patients were those who required a total colonoscopy to the caecum, and were older than 20 years. Patients were excluded if they had: (1) severe chronic renal failure (creatinine >1.5 mg/dL); (2) severe congestive heart failure (New York Heart Association class III or IV); (3) history of percutaneous coronary intervention or coronary artery bypass grafting for the treatment of myocardial infraction in the past 3 months; (4) blood electrolyte abnormalities; (5) chronic active inflammatory bowel disease; (6) resected digestive tract (except appendectomy); (7) possibility of gastrointestinal obstruction, perforation or disorders of gastric emptying; (8) toxic megacolon; (9) chronic constipation (defection <3 times a week); (10) history of hypersensitivity to PEG or any other ingredient in the products used in this study; (11) pregnancy or breast feeding; or (12) diagnosis of advanced colorectal cancer.
Study design
This was an observer-blinded, prospective, randomised study. Patients were randomised to receive one of the following three cleansing regimens using a computer-generated random number list:
The PEG-Asc-S regimen involved intake of 1.5 L of hypertonic PEG-Asc (Moviprep, Ajinomoto Pharmaceutical Co) with one-split dosing (1 L of PEG-Asc+0.5 L of water, then 0.5 L of PEG-Asc+0.25 L of water).
The PEG-Asc-M regimen involved intake of 1.5 L of PEG-Asc with four-split dosing (five lots of (0.3 L of PEG-Asc+0.15 L of water)).
The PEG-ES regimen involved intake of 2.25 L of isotonic PEG-ES solution (Ajinomoto Pharmaceutical Co) with no-split dosing.
A detailed description of the composition of PEG-Asc and PEG-ES is given in the online supplementary table S1.
supplementary data
As shown in figure 1, the total volume which the patient is required to drink was 2.25 L for all three regimens. Dosing was performed in our hospital under the guidance of endoscopy-specific nurses, and colonoscopy was performed on the same day by qualified colonoscopists with at least 4 years of experience who were blinded to the cleansing regimens. On the day before colonoscopy, patients did not eat a prepacked low-residue diet, and were instead instructed to have an ordinary supper but without seaweed, mushrooms or fruits such as kiwifruit that contain seeds that are not easily digested. Patients were also recommended to have plenty of water or tea but not to have laxatives. On the day of colonoscopy, patients were instructed not to have breakfast.
Intake schedules for the PEG-Asc-S, PEG-Asc-M and PEG-ES regimens. The PEG-Asc-S regimen was 1.5 L of Moviprep with one-split dosing. The PEG-Asc-M regimen was 1.5 L of Moviprep with four-split dosing. The PEG-ES regimen was 2.25 L of PEG-ES with no split. PEG-Asc, polyethylene glycol-ascorbic acid solution; PEG-ES, polyethylene glycol-electrolyte solution.
To compare the dehydration effects between regimens, haematocrit (Ht), red blood cell values and haemoglobin were measured just before dosing (baseline), at the end of dosing and at the end of colonoscopy. Biochemical tests were also performed just before the start of dosing (baseline), at the end of dosing and at the end of colonoscopy. The percentage changes in Ht, haemoglobin, Na, etc, from baseline to the end of dosing and from baseline to the end of colonoscopy were defined as 100(D−B)/B and 100(C−B)/B, respectively, where B is the value at baseline, D is the value at the end of dosing and C is the value at the end of colonoscopy.
Assessment of bowel cleansing
The efficacy of bowel cleansing was evaluated based on video-stored images of procedures by an independent outside committee blinded to the treatment allocations. The degree of bowel cleansing was rated for each of the following segments: caecum/ascending colon, transverse colon, descending colon, sigmoid colon and the rectum.1 ,17 The overall quality of cleansing was determined based on the assessment of the individual segments as excellent (clean in all segments), good (removable residue in one or more segments), poor (non-removable solid stool in one or more segments) or missing (image data were accidentally not recorded or were of poor quality). Excellent and good are successful grades. Unsuccessful grades of C (poor) and D (bad) in the Harefield Cleansing Scale were combined as poor in the present study.1 ,17
Statistics
This study was designed to demonstrate that PEG-Asc given as a one-split dose and as a four-split dose was at least as safe as PEG-ES in terms of the proportion of Ht in the normal range. Previously, 94% of participants who received PEG-ES showed Ht in the normal range at the end of dosing (unpublished results of the phase III Japanese clinical trial of PEG-Asc, provided by Ajinomoto Pharmaceutical Co, Tokyo. A non-inferiority design was therefore used, with non-inferiority defined as occurring if the lower limit of the two-sided 95% CI for the difference in occurrence rates of normal Ht between the treatment groups was <−10%. A sample size of 93 participants per group was estimated to be required, with a two-sided significance level of α=0.05, equivalent limit of non-inferiority of 0.10, and a power of 80%.
The differences in demographic properties and reason for colonoscopy between the three groups were analysed using Cochran-Mantel-Haenszel tests or analysis of variance (ANOVA). The difference in the efficacy distribution (excellent, good, poor or missing) between two bowel preparations was evaluated using the Wilcoxon rank-sum test (two samples). The difference in time necessary for bowel preparation between different groups was assessed using ANOVA. The mixed-effects model was used for the differences in laboratory test values between baseline and at the end of dosing, and between baseline and at the end of colonoscopy in the same group. The mixed-effects model was used for the differences between groups at the end of dosing and at the end of colonoscopy. The criterion for statistical difference was p<0.05.
Results
Study disposition is shown in figure 2. A total of 310 patients were randomised to the PEG-Asc-S group (n=107), the PEG-Asc-M group (n=99) or the PEG-ES group (n=104). After randomisation, two patients in the PEG-Asc-S group withdrew their consent, two patients in the PEG-ES group had a major protocol deviation, and three patients in the PEG-Asc-S group and two patients in the PEG-ES group discontinued dosing because of nausea due to stomach fullness. One patient in the PEG-Asc-M group had nausea but completed dosing. Therefore, a final total of 301 patients were analysed (102 in the PEG-Asc-S group, 99 in the PEG-Asc-M group and 100 in the PEG-ES group).
Flow diagram showing randomisation of patients into treatment groups. PEG-Asc, polyethylene glycol-ascorbic acid solution; PEG-ES, polyethylene glycol-electrolyte solution.
Table 1 shows the demographic characteristics and reasons for colonoscopy. These characteristics and reasons were not significantly different between the three groups.
Demographic characteristics and reasons for colonoscopy
Efficacy
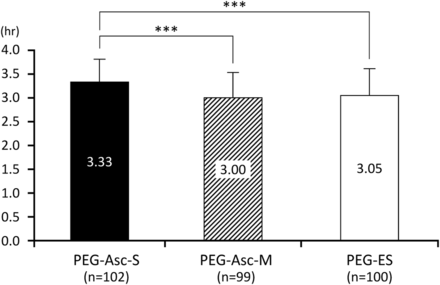
The time necessary for the completion of dosing is shown in figure 3. The time for bowel preparation in the PEG-Asc-S group (3.33±0.48 hours, n=102) was significantly longer than in the PEG-Asc-M group (3.00±0.53 hours, n=99) and the PEG-ES group (3.05±0.56 hours, n=100; p<0.001 for both comparisons). There was no significant difference in bowel preparation time between the PEG-Asc-M and the PEG-ES groups.
The time necessary for the completion of dosing. ***p<0.001. PEG-Asc, polyethylene glycol-ascorbic acid solution; PEG-ES, polyethylene glycol-electrolyte solution.
The distribution of overall cleansing evaluation as excellent, good, poor or missing in each group is shown in table 2. The distribution did not significantly differ between any two of the groups; however, there was a tendency for there to be more ‘excellent’ ratings in the PEG-Asc-S (76%) and PEG-Asc-M (75%) groups than in the PEG-ES group (65%). Bowel preparation was successful (the combined ratings of ‘excellent’+‘good’) for 96%, 94% and 95% of patients in the Modi-S, Modi-M and PEG-ES groups, respectively.
Efficacy of the PEG-Asc-S, PEG-Asc-M and PEG-ES regimens
The number of polyps/adenomas per person (mean±SD, range) in the PEG-Asc-S, PEG-Asc-M and PEG-ES groups were 2.97±2.92 (0–15), 3.32±3.51 (0–19) and 3.53±3.27 (0–17). There was no significant difference between any two groups.
Dehydration parameters—Ht, haemoglobin and red blood cells
Table 3 shows the Ht values at baseline, at the end of dosing and at the end of colonoscopy. Ht in all three regimens was significantly increased at the end of dosing compared with baseline (p<0.001 for all three comparisons), indicating occurrence of physiological dehydration during the cleansing.
Results of blood and biochemical tests (mean±SD)
The percentage changes in Ht from baseline to the end of dosing were 3.53±3.32%, 4.11±3.07% and 1.31±3.01% in the PEG-Asc-S, PEG-Asc-M and PEG-ES groups, respectively (figure 4). The extent of these positive percentage changes reflects physiological dehydration during the cleansing. The extent of dehydration in the PEG-Asc-S and PEG-Asc-M groups was significantly greater than that in the PEG-ES group (p<0.001 for both comparisons). The difference in level of dehydration between the PEG-Asc-S and PEG-Asc-M groups was not significant.
Percentage changes in levels of haematocrit, red blood cells and haemoglobin from baseline to the end of dosing (left) and from baseline to the end of colonoscopy (right). Baseline is the value measured before dosing. Significances of percentage changes in red blood cells and haemoglobin between the PEG-Asc-S and PEG-ES groups, and that between the PEG-Asc-M and PEG-ES groups were the same as those of haematocrit (not shown here; detailed description given in the online supplementary tables S2 and S3). ***p<0.001. PEG-Asc, polyethylene glycol-ascorbic acid solution; PEG-ES, polyethylene glycol-electrolyte solution.
The percentage changes in Ht from baseline to the end of colonoscopy in the PEG-Asc-S and PEG-Asc-M groups (−0.64% and −0.45%, respectively) were significantly smaller than that in the PEG-ES group (−2.47%) when compared in absolute values. The difference between the percentage change from baseline to the end of dosing and that from baseline to the end of colonoscopy would reflect the extent of rehydration during colonoscopy, which was 4.17±0.34%, 4.56±0.35% and 3.78±0.32% in the PEG-Asc-S, PEG-Asc-M and PEG-ES groups, respectively. No significant difference was observed in rehydration between any two groups.
The number of participants who had Ht values within the normal ranges of 39.0–50.4% for males and 34.0–44.0% for females at baseline, and Ht values exceeding the upper limits of normal at the end of dosing was six, three and zero in the PEG-Asc-S, PEG-Asc-M and PEG-ES groups, respectively. The Ht of these six patients in the PEG-Asc-S group and two of these patients in the PEG-Asc-M group returned to normal at the end of colonoscopy.
Three patients (one patient from each of the PEG-Asc-S, PEG-Asc-M and PEG-ES groups) had Ht values exceeding the upper limits of normal at baseline. Ht values at baseline, at the end of dosing and at the end of colonoscopy were 60.6, 61.8 and 60.3, respectively, for a male patient in the PEG-Asc-S group, 50.7, 52.0 and 50.0, respectively, for a male patient in the PEG-Asc-M group, and 47.3, 48.2 and 47.4, respectively, for a female patient in the PEG-ES group. The male patient in the PEG-Asc-S group was diagnosed with polycythaemia after this study.
Next, the percentage changes in haemoglobin and red blood cells were studied as the other possible dehydration parameters. Figure 4 shows these percentage changes from baseline to the end of dosing and from baseline to the end of colonoscopy in the PEG-Asc-S, PEG-Asc-M and PEG-ES groups (detailed description in online supplementary tables S2 and S3). These three parameters showed very similar percentage changes in each group. The levels of percentage changes at the end of dosing in the PEG-Asc-S and PEG-Asc-M groups were significantly greater than that in the PEG-ES group (p<0.001 for both comparisons). This indicates that haemoglobin and red blood cell levels, as well as Ht values, are good indicators of dehydration and rehydration during colon cleansing and colonoscopy, respectively.
Electrolytes, blood urea nitrogen and creatinine
Table 3 shows the levels of blood electrolytes, blood urea nitrogen and creatinine. The percentage changes in blood Na from baseline to the end of dosing and from baseline to the end of colonoscopy were <0.5% in PEG-Asc-S, PEG-Asc-M and PEG-ES groups. Hyponatraemia in cleansing studies has been previously reviewed.18 In the present study, one patient in each group had hyponatraemia (Na<138 mEq) at the end of colonoscopy. In these three patients, the respective values of Na at baseline, at the end of dosing and at the end of colonoscopy were 136, 136 and 136 in the patient from the PEG-Asc-S group, 138, 139 and 135 in the patient from the PEG-Asc-M group, and 130, 130 and 131 in the patient from the PEG-ES group. That is, one patient in the PEG-Asc-M group showed a decrease in Na at the end of colonoscopy compared with baseline, and the other two patients maintained the low Na values recorded at baseline until the end of colonoscopy. These patients did not show any abnormal clinical presentations.
The percentage changes in other electrolytes such as K, Cl and Ca from baseline to the end of dosing and from baseline to the end of colonoscopy were <3% in all groups. The percentage changes in creatinine from baseline to the end of dosing and from baseline to the end of colonoscopy were <2% in all groups.
Figure 5 shows the percentage changes in blood urea nitrogen from baseline to the end of dosing and from baseline to the end of colonoscopy (detailed description in online supplementary table S4). These percentage changes were negative in all three groups. Percentage changes in blood urea nitrogen from baseline to the end of dosing in the PEG-Asc-S and PEG-Asc-M groups were significantly smaller than that in the PEG-ES group when compared in absolute values (p<0.001 and p<0.05, respectively).
Percentage changes in BUN from baseline to the end of dosing and from baseline to the end of colonoscopy. *p<0.05, ***p<0.001 (detailed description given in the online supplementary table S4). BUN, blood urea nitrogen; PEG-Asc, polyethylene glycol-ascorbic acid solution; PEG-ES, polyethylene glycol-electrolyte solution.
Discussion
In the present study, three lower volume bowel cleansing regimens were explored; 1.5 L of PEG-Asc+0.75 L of water with one-split dosing (PEG-Asc-S) or four-split dosing (PEG-Asc-M), and 2.25 L of PEG-ES with no-split dosing (PEG-ES). The success rates of bowel preparation with these three regimens were more than 94%. Although there was a tendency for more ‘excellent’ ratings in the PEG-Asc-S (76%) and PEG-Asc-M (75%) groups than in the PEG-ES group (65%), the distributions of overall cleansing evaluation of excellent, good and poor between three regimens were not significantly different. Compliance was considered to be good because patients did not have to eat a prepacked low-residue diet, take laxative medicines or take a part or full dose of the cleansing regimen on the day before colonoscopy. Fasting on the morning of the colonoscopy was very important. Guidance for patients to follow the exact intake schedule may be another important aspect that resulted in the very high success rates using these lower volume regimens.
Generally, a shorter time for bowel preparation is preferable because it decreases the burden on patients. The PEG-Asc-S regimen with one-split dosing is recommended in Japan. The time necessary for completion of dosing with hypertonic PEG-Asc-S was significantly longer than that with isotonic PEG-ES. The present new PEG-Asc-M regimen with four-split dosing overcame the inconvenience of the long time required with PEG-Asc-S.
Another advantage of the four-split dosing is that it appears to have prevented the occurrence of severe nausea. Three patients in the PEG-Asc-S group and two patients in the PEG-ES group had nausea due to fullness of the stomach and therefore discontinued dosing; however, no patient in the PEG-Asc-M group had nausea that resulted in the discontinuation of dosing.
The extent of dehydration that occurs during bowel cleansing with hypertonic PEG-Asc is supposedly greater than that with isotonic PEG-ES; however, this has so far not been adequately investigated. Furthermore, it has been suggested that isotonic PEG-ES causes little fluid exchange across the colonic mucosal membrane, thereby limiting the potential for systemic electrolyte disturbance.15 In the present study, the three blood test parameters of Ht, red blood cell and haemoglobin were significantly greater at the end of dosing than at baseline. The mean percentage changes in these three parameters from baseline to the end of dosing in the PEG-Asc-S group and PEG-Asc-M groups were significantly greater than in the PEG-ES group. These changes in Ht reflect physiological dehydration, as no patients showed pathological dehydration-related adverse effects such as cognitive dysfunction.19 Cleansings with hypertonic PEG-Asc with one-split and four-split dosing have greater physiological dehydration effects than isotonic PEG-ES. However, even isotonic PEG-ES has physiological dehydration effects during cleansing.
The Ht changes from baseline to the end of colonoscopy in the PEG-Asc-S, PEG-Asc-M and PEG-ES groups (−0.65%, −0.54% and −2.47%, respectively) indicate that rehydration occurred during colonoscopy. As patients did not have breakfast on the day of colonoscopy, the Ht value at baseline would reflect a slightly dehydrated state. The value of −2.47% in the PEG-ES group would reflect the non-dehydrated state at the end of colonoscopy. In other word, patients in the PEG-Asc-S and PEG-Asc-M groups at the end of colonoscopy were 2% more dehydrated than those in the PEG-ES group. This suggests that patients treated with PEG-Asc should intake additional water after colonoscopy to cope with the physiological dehydration.
The percentage changes (in absolute values) of blood urea nitrogen from baseline to the end of dosing in the PEG-ES group were significantly greater than that in the PEG-Asc-S and PEG-Asc-M groups, and the difference between the PEG-Asc-S and PEG-Asc-M groups was not significant. The cleansing almost completely evacuates stools from the bowel, which results in a decrease in blood urea nitrogen, possibly with the same degree of decrease in all three groups. Dehydration during the cleansing would increase blood urea nitrogen, with larger increases in the PEG-Asc-S and PEG-Asc-M groups compared with the PEG-ES group. These defecation and dehydration effects may explain the trend of the percentage changes in blood urea nitrogen at the end of dosing in the three groups.
In conclusion, the use of lower volume 1.5 L PEG-Asc with one-spilt and four-split dosing and 2.25 L PEG-ES with no-split dosing resulted in very high success rates in bowel cleansing with no serious adverse effects. The new regimen of 1.5 L PEG-Asc with four-split dosing significantly shortened bowel cleansing time compared with one-split dosing. The Ht, red blood cell and haemoglobin values are sensitive dehydration indicators. The physiological dehydration associated with PEG-Asc was significantly greater than that with PEG-ES at the end of dosing and even patients cleansed with isotonic PEG-ES showed significant physiological dehydration at the end of dosing. Patients treated with PEG-Asc are suggested to intake additional water after colonoscopy.
Acknowledgments
The authors would like to thank Tokiko Itoh, Akita Red Cross Hospital for her assistance with data management and Masafumi Nomura, Center of Gastroenterology, Teine Keijinkai Hospital, Sapporo, Japan and Hibiki Ohtani, Division of Gastroenterology, Japan Community Health Care Organization, Hitoyoshi Medical Center, Hitoyoshi, Kumamoto, Japan for their evaluation of cleansing scores.
References
Footnotes
Contributors HY, HM, KY, RT, EH, YT, MN, RH and YY contributed to the study's concept and designs. All authors collected the clinical data. HY, HM and KY analysed and interpreted the data. HY drafted the manuscript. HY provided administrative support. All authors contributed to the critical revision of the manuscript.
Funding This work was supported by Akita Red Cross Hospital, Akita, Japan. This work was also supported by a supporting grant from Ajinomoto Pharmaceutical Co, Tokyo, Japan, to Digestive Disease Center, Akita Red Cross Hospital, Akita, Japan.
Competing interests HY received grant support for this study from Ajinomoto Pharmaceutical Co and lecturing fees from Eisai Co, Takeda Pharmaceutical Co, Ajinomoto Pharmaceutical Co, KYORIN Pharmaceutical Co, DAIICHI SANKYO Co, AstraZeneca K. K., ZERIA Pharmaceutical Co, Otsuka Pharmaceutical Co, Olympus medical science sales Co and Olympus Co.
Patient consent Obtained.
Ethics approval Institutional Review Board at Akita Red Cross Hospital, Akita, Japan.
Provenance and peer review Not commissioned; externally peer reviewed.
Data sharing statement The authors included an online supplementary file with additional data.