Article Text
Abstract
Background Percutaneous endoscopic gastrostomy is a commonly used endoscopic technique where a tube is placed through the abdominal wall mainly to administer fluids, drugs and/or enteral nutrition. Several placement techniques are described in the literature with the ‘pull’ technique (Ponsky-Gardener) as the most popular one. Independent of the method used, placement includes a ‘blind’ perforation of the stomach through a small acute surgical abdominal wound. It is a generally safe technique with only few major complications. Nevertheless these complications can be sometimes life-threatening or generate serious morbidity.
Method A narrative review of the literature of major complications in percutaneous endoscopic gastrostomy.
Results This review was written from a clinical viewpoint focusing on prevention and management of major complications and documented scientific evidence with real cases from more than 20 years of clinical practice.
Conclusions Major complications are rare but prevention, early recognition and popper management are important.
- gastrostomy
- endoscopic gastrostomy
- enteral nutrition
Data availability statement
No data are available. Data from illustrations are deidentified.
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.
Statistics from Altmetric.com
Introduction
The first and still most widely used ‘pull’ technique to introduce a percutaneous endoscopic gastrostomy (PEG) was developed by Ponsky-Gardener in the early 1880s.1 Three years later, Sachs-Vine described their ‘push’ technique. This is similar to the ‘pull’ technique but here the guidewire stays in place and the PEG is pushed over the guidewire through the mouth, oesophagus, stomach, and finally comes out through the anterior abdominal wall.2 In 1984, Russel used a peel-away introducer sheath and dilatator over a guidewire to enter the stomach. During this ‘introducer technique’, the guidewire and dilatator (trocar) are removed and through the remaining sheath, a balloon-tipped catheter is inserted.2 3 A potential problem with introducing the dilatator was that the stomach was being pushed away from the anterior abdominal wall. To secure the abdomen to the anterior gastric wall, Wu et al described in 1987 a modification of this technique by performing gastropexy: under endoscopic guidance they inserted a special needle to place nylon anchoring devices with T-fasteners at the end.2 4
If patients require enteral access for >4 to 6 weeks, a PEG is recommended by international guidelines.5 A PEG-tube can serve as a vehicle for liquid feeding formulas, fluid and/or liquid medications into the stomach but can also be used for decompression, drainage or management of gastric volvulus.6
It is retained in position by an internal and external fixation device, fixator or bumper. The internal bumper holds the device securely inside the stomach. It may be in the form of a flange, dome, string, basket or balloon. The external bumper may be in the form of a triangle circle or other shape, can be soft or hard and secures the gastrostomy tube externally against the abdominal wall, limiting unnecessary tube movement and leakage of gastric contents.7
PEG tube insertion is usually considered a safe procedure, however, complications can occur with a variable rate based on the study population. These complications can be classified as minor or major.8 In a large retrospective follow-up study of 1625 patients after PEG placement the procedural, 30-day and overall mortality rates were 0.2, 2.4 and 14.0%, respectively.9 In a recent retrospective analysis in 465 PEG patients major complications occurred in 10 (2.0%) patients. There were no differences in the age or body mass index of patients with either minor or major complications.10 In this narrative review, existing evidence of major post procedural PEG complications is explored while focusing on prevention and management. Furthermore, the evidence is documented with real cases from more than 20 years of clinical practice.
Cardiopulmonary events
Cardiopulmonary adverse events related to sedation and analgesia account for much as 60% of upper gastrointestinal endoscopy adverse events. These events can be minor (eg, changes in oxygen saturation or heart rate) or major (eg, respiratory arrest and aspiration pneumonia). Risk factors can be divided in patient-related factors (eg, advanced age, polymorbidity, severe systemic disease, head and neck cancer) and procedure-related factors (eg, prolonged procedure, difficulty with intubation if needed). Patients should provide informed consent before administering sedation which include a discussion about benefits, risks, limitations as well as possible alternatives to the sedation plan.11 As example in patients with significant respiratory compromise, (nasal) unsedated PEG placement could be an alternative.12 13 Special attention is required for patients who lack decision-making capacity, such as dementia, acquired brain injury or an intellectual disability. Decisions about PEG placement could have major implications for life expectancy, quality of life and survival and the potential to prolong suffering. Recent guidelines consider patient age, the presence of stroke as an indication and preprocedural nutritional and inflammatory status as risk factors for early and long-term PEG-related mortality.14 In the entire PEG care pathway, but mainly in recognising the complexities of making decisions about PEG placement, the presence of a Nutritional Support Team can offer an important added value where wider issues can be discussed in detail and depth in an ongoing process.15 Extensive preprocedural preparation and assessment before sedation and intraprocedural patient monitoring during (propofol) sedation are essential prerequisites11:
Prevention
Evaluate risk for sedation and potential problems related to pre-existing medical conditions.
Perform a focused physical examination on elements that could impact sedation (eg, history of stridor, sleep apnoea, former adverse reaction to sedation or anaesthesia, oral or neck abnormalities, tobacco use).
Presence of a sedation team with appropriate education and training with at least one person qualified in advanced life support skills throughout (propofol) sedation.
Provide age-appropriate equipment for airway management and resuscitation.
Management options
Administer supplemental oxygen during sedation.
Monitor oxygenation by pulse oximetry, electrocardiography and intermittent blood pressure measurement.
Visually assess ventilator activity, level of consciousness and discomfort.
Consider the use of capnography which has been demonstrated to detect depressed airway respiratory activity before transient hypoxaemia.
Apply airport support manoeuvres if necessary (eg, chin lifts, jaw thrusts, nasal airways).
Buried bumper syndrome
Description
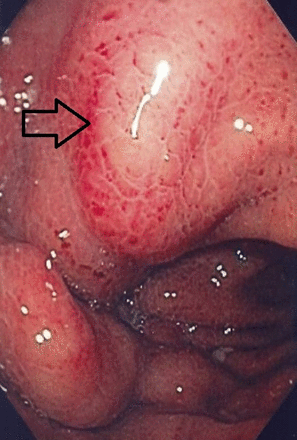
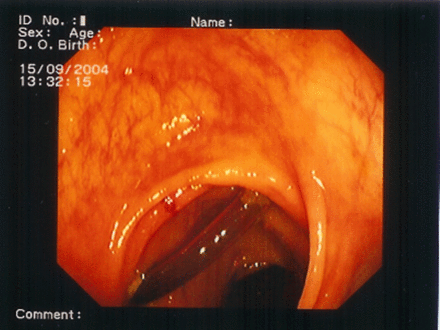
A buried bumper syndrome (BBS) means that the internal fixation device has migrated and becomes lodged anywhere between the gastric wall and the skin along the PEG tract. BBS is a usually long-term complication where the gastric mucosa gradually covers the internal bumper but has also been reported to occur as early as 3 weeks after PEG placement (see figure 1). Incidence is estimated at around 1% (0.3%–2.4%). Excessive compression of tissue between the external and internal fixation device of the gastrostomy tube is considered as the main etiological factor leading to BBS.16 17 It can manifest itself with minor complications such as feeding intolerance, peristomal leakage, pain, swelling at the site of tube insertion, stoma infection and tube obstruction, whereas major complications include peritonitis, perforation, gastrointestinal bleeding, abdominal wall abscesses or sepsis.18
Complete buried bumper (see arrow).
The most alarming symptom is that the tube cannot be moved inwards. Also with a percutaneous endoscopic transgastric jejunostomy (PEG-J), BBS can occur. In one study in paediatric patients, those with a PEG-J developed significantly more BBS than those with PEG tubes.19 BBS is a serious complication which can be prevented with proper after care.5 7 Treatment depends on patient condition, type of PEG-tube and degree of migration (in or outside the stomach or complete versus incomplete covering).14 16–23
Prevention
Avoid excessive tension of the external bumper against the skin. Immediately after placement of the PEG, the external bumper should be subjected to very low traction, without tension.
Rotate the tube daily but importantly: move the tube inwards (at least 2 cm, up to 10 cm) once the gastrostomy tract has been healed (after about 7–10 days).
After mobilisation, return the tube to its initial position with some free distance (1–2 cm) between the skin and the external bumper.
Management options
Apply a careful preintervention risk assessment.
Confirm diagnosis by gastroscopy.
Consider a conservative approach in patients with a poor prognosis, frailty and/or very high iatrogenic risk for invasive procedures. This approach implies cutting the tube and leaving the internal bumper in situ followed by eventually inserting a new PEG adjacent to the first site.
Apply external traction and remove (if a collapsible bumper was used).
Remove endoscopically (if not totally covered) by cutting the old tube short and introduce through it a guidewire which afterwards is grasped or snared by the endoscope and pulled out. Pull a new gastrostomy in and push the old bumper out of the abdominal wall.
Remove endoscopically by using a needle knife or guide-wire papillotome.
Consider using The Flamingo device which has recently been introduced as the first tool specifically designed to remove a completely buried bumper.
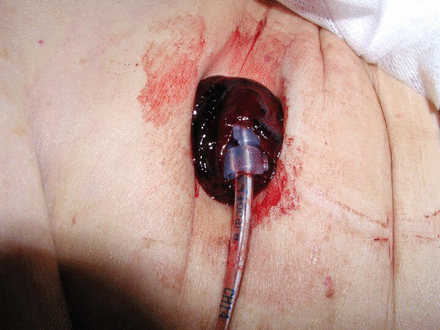
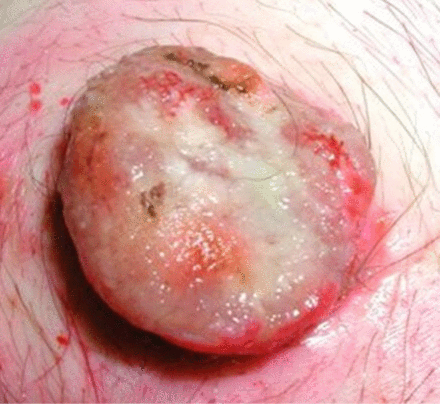
Remove surgically (laparoscopically or laparotomy) (see figure 2).
Buried bumper after surgical removal.
Bleeding
Description
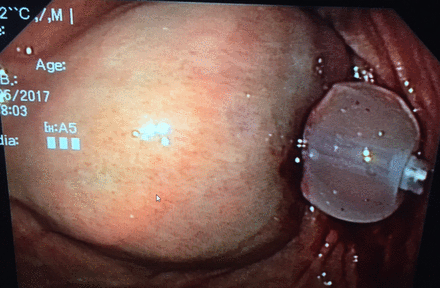
Significant bleeding during percutaneous enteral access is uncommon with an incidence of 2,67%.24 Most bleeding represents disruption of superficial blood vessels arising from the tube tract due to the inserted trocar or needle (see figure 3). Ulceration with subsequent bleeding may also occur in the stomach on the posterior wall opposite the PEG or underneath the internal bumper (see figure 4). Abdominal wall and rectus sheath haematomas are also described.25 26 Cutaneous and intraluminal bleeding are immediately recognised but there are case reports of aortic perforation, gastric artery injury, splenic or mesenteric vein injuries (massive retroperitoneal bleeding) and this may remain occult.27 28 Therefore vital parameters (unexplained tachycardia/hypotension) should be monitored up to 2 hours after procedure. Guidelines, including a very recent one from The European Society of Gastrointestinal Endoscopy, strongly recommend that percutaneous tube placement should be considered as a procedure with high haemorrhagic risk and that in order to reduce this risk, specific guidelines for antiplatelet or anticoagulant use should be followed strictly.29 30
Disruption of a superficial blood vessel arising from the tube tract with bleeding which stopped after tightening the external bumper.
Stomach wall bleeding/haematoma underneath the internal bumper (balloon).
Prevention
Correct coagulopathy before the procedure: recommended threshold for the procedure is a platelet count of 50 000/µL and International Normalized Ratio (INR) <1.5.
Patients on P2Y12 receptor antagonist antiplatelet agents with low thrombotic risk:
Discontinue P2Y12 receptor antagonists (clopidogrel, prasugrel, ticagrelor) 5 days before the procedure.
Low-dose aspirin is no contraindication and should not be discontinued.
Patients on P2Y12 receptor antagonist antiplatelet agents with high thrombotic risk (coronary artery stents):
Continue aspirin and liaise with a cardiologist about the risk/benefit of discontinuation of P2Y12 receptor antagonist.
Patients on direct oral anticoagulants (DOAC):
Discontinue DOAC (dabigatran, rivaroxaban, apixaban, edoxaban)≥48 hours before the procedure.
For dabigatran with Creatinine Clearance (CrCl) estimated Glomerular filtration rate (eGFR) 30–50 mL/min take last dose of drug 72 hours before procedure.
Patients on warfarin with low-risk condition:
Stop warfarin 5 days before the procedure.
Check International Normalized Ratio (INR) prior to procedure to ensure International Normalized Ratio (INR) <1.5.
Restart warfarin evening of procedure with usual daily dose.
Check International Normalized Ratio (INR) 1 week later to ensure adequate anticoagulation.
Patients on warfarin with high-risk condition:
Stop warfarin 5 days before the procedure.
Start Low Molecular Weight Heparine(LMWH) 2 days after stopping warfarin.
Give last dose of Low Molecular Weight Heparine (LMWH) ≥24 hours before the procedure.
Restart warfarin evening of procedure with usual daily dose.
Continue Low Molecular Weight Heparine (LMWH) until International Normalized Ratio (INR) adequate.
Management options
For superficial bleeding: tighten the bumper to apply direct pressure but don’t forget to release it within 24–48 hours to avoid pressure damage.
In more complicated cases or persisting bleeding: repeat endoscopy.
Rarely surgical intervention will be necessary.
Tube dislodgement
Description
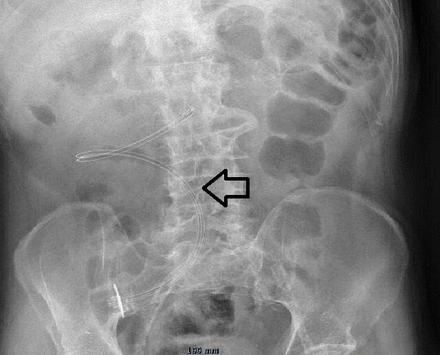
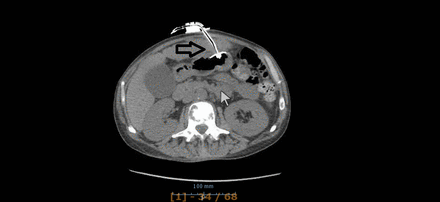
Tube dislodgment can occur when the gastrostomy tube either slides in or out of the gastrointestinal tract. Inadvertent tube removal usually is the result from (excessive) external traction (pulling) on the tube for example, during episodes of agitation, confusion or aggression or spontaneously when the internal balloon deflates. If this happens when the stomach serosa has not had the time to adhere to the parietal peritoneum, there is a risk of leakage into the peritoneal cavity. On the other hand when the tube slides too far into the gastrointestinal tract, it can obstruct the pyloric region (gastric outlet obstruction) or even migrate further in the duodenum or gut (see figures 5 and 6). Typical symptoms are abdominal cramps, nausea or vomiting. This happens when the external bumper migrates away from the abdominal wall.8 Early accidental dislodgement is reported in 0.6%–4.0%. In a large retrospective analysis a total of 563 PEGs were identified of which 72 got accidentally dislodged: the early dislodgement rate was 4.1% (23/563), 49 occurred after discharge in rehabilitation or nursing facilities. The vast majority required an emergency department visit, a surgical consultation, a replacement gastrostomy tube and/or a radiographic confirmation of tube positioning, resulting in costs totalling an average of US$1200.31 Because it is not uncommon and expensive, prevention and proper management are important.8 14 32
On the left side a PEG which became dislodged and migrated into the duodenum which resulted in a gastric outlet obstruction. On the right side after retraction to its initial position. PEG, percutaneous endoscopic gastrostomy.
A patient who dismantled his PEG with loss of the clamp, external bumper and feeding connector. The PEG migrated into the gut and could be removed rectally after 7 days. PEG, percutaneous endoscopic gastrostomy.
Prevention
Consider the use of abdominal binders, an elastic bandage and clinical restraints to prevent access to the tube.
Tailor the tube at a length that allows adequate care but also restricts access.
Assure that the external bumper is appropriately positioned (with too much free space the internal bumper can migrate forward).
Management options
For tubes in place >4 weeks:
Apply blind bedside tube replacement through the tube tract which is generally safe and easy. It should be attempted as soon as possible to prevent the tube tract from closing. A similarly sized Foley catheter is commonly available and makes for a great temporary replacement PEG. If gastric contents can be aspirated with a pH of ≤5.5 and the tube flushes problem-free with sterile water, a confirmatory radiographic study is not necessary
For tubes in place <4 weeks:
‘Blind’ tube reinsertion should be avoided. The patient should be monitored clinically and broad-spectrum antibiotics should be administered in symptomatic patients. A new PEG should be placed once the initial tract has healed.
Manage the patient by nasogastric aspiration, broad-spectrum antibiotics, and parenteral nutrition if unable to replace the PEG immediately.
Monitor for signs of peritonitis.
Consider surgical exploration if the patient demonstrates signs of peritoneal leakage and early peritonitis.
Gastric erosion and ulcers
Description
Gastrostomy tubes with an internal retention disc are at risk for progressive disc migration into and through the gastric wall which can lead to gastric ulcer formation and erosion. This severe delayed complication results from prolonged excessive traction on the retention disc which can be complicated with (gastrocolic or gastrocolocutaneous) fistula formation.33 This complication can be avoided with improved tube aftercare and education.7 14
Prevention
Rotate the tube daily but more importantly, move the tube inwards (at least 2 cm, up to 10 cm) once the gastrostomy tract has been healed (after about 7–10 days).
Return the tube after mobilisation to its initial position with some free distance (1–2 cm) between the skin and the external bumper.
Management options
Surgical resection of the disc and fistula repair if needed.
Necrotising fasciitis
Description
Necrotising fasciitis (necrosis of the fascia layers) is an uncommon and potentially fatal soft tissue infection involving skin, subcutaneous tissue and muscle, usually caused by toxin-producing virulent bacteria. Immunocompromised, malnourished and patients with diabetes have an increased risk. Symptoms include erythema, oedema and the development of bullae. Several aerobic and anaerobic pathogens may be involved, including Bacteroides, Clostridium, Peptostreptococcus, Enterobacteriaciae, Proteus, Pseudomonas and Klebsiella, but group A haemolytic streptococcus and Staphylococcus aureus, alone or in synergism, are the initiating infecting bacteria.34 Case series after PEG placement are reported in the literature.35 36
Prevention
Use intravenous antibiotic (penicillin-based or cephalosporin-based) administration 30 min before the PEG procedure.37
Apply standard measures for infection prevention including aseptic preparation of the surgical field and preoperative handwashing/disinfection.38
Use of a mouthwash with an oral chlorhexidine solution to reduce bacterial burden.38
Avoid traction on the gastrostomy and excessive pressure between the internal and external bumper.
Management options
Antibiotics and emergent surgical debridement and sometimes multiple repeated debridement and reconstructive abdominal wall surgery.34
Pneumoperitoneum peritonitis and peritonitis
Description
Pneumoperitoneum peritonitis
With high intragastric air pressure during endoscopy, air may escape during needle puncture and the passage of the PEG tube through the abdominal wall, resulting in free intra-abdominal air. Transient subclinical pneumoperitoneum after PEG placement depending on the study is a common finding and is generally not considered as a complication if there are no peritoneal signs.8 39 Nevertheless a small minority of patients can develop signs of peritonitis which can be devastating if not early recognised.40 In a retrospective review of 722 patients after PEG placement, 39 patients found to have free air of which the majority (33 patients) had ‘benign pneumoperitoneum’. Of the remaining six patients, five had clinical symptoms of peritonitis.41 In a large study in 281 Intensice Care Unit (ICU) patients with radiologic imaging, pneumoperitoneum was found in 16% (45/281). Eight patients were found to require either surgical or endoscopic emergent intervention post-PEG.42
Prevention
Consider the use of carbon dioxide insufflation instead of ambient air (in a randomised controlled trial this significantly reduced the frequency of postprocedural pneumoperitoneum).43
Management options
If benign: apply conservative treatment (pneumoperitoneum is usually self-limiting within 72 hours of PEG insertion).
If peritoneal signs: further investigation and/or early actions are required (eg, CT-scan)
Peritonitis
Description
Fullblown peritonitis presents as abdominal pain, leucocytosis, ileus and fever. If after PEG placement the stomach serosa has not had time to adhere to the parietal peritoneum (inadequate sealing) gastric content can leak into the peritoneal cavity and can result in consequent peritonitis.14 44 Patients with diabetes and malnutrition are at risk. In the case of tube dislodgment (see also complication ‘tube dislodgement’) within the first 4 weeks of its insertion, there is a risk of peritonitis. Ascites can also preclude adequate seal and lead to continued ascites leak and predispose the patient to bacterial peritonitis. PEG placement is potentially feasible if ascites is adequately drained through paracentesis prior to placement and the patient is kept dry until an appropriate seal is formed.44 Also some cases have been reported where (forced) replacement of G-tubes in immature stoma tracts, resulted in intraperitoneal placement and peritonitis.45
Prevention
See prevention in ‘necrotising fasciitis’.
Perform a proper risk assessment in patients with (mild) ascites.
Management options
Interrupt enteral nutrition, uncap the tube and connect it to a drainage bag in order to relieve abdominal distension and pain (ileus).
Perform abdominal imaging.
Administrate intravenous antibiotics.
Colonic injury
Description
Injury of a bowel (mostly the transverse colon) can accidentally happen.46 If the colon loop lies in close proximity to the stomach or even overlies the stomach, this rare complication can occur. In most cases the PEG will penetrate through the colon prior to entering the stomach (see figure 7). Less commonly it can also happen with progressive erosion and migration of the tube into the juxtaposed colon. Patients may develop colocutaneous or gastrocolic fistulas that become evident only at the time of tube removal. Insertion of a PEG into the colon may present as severe diarrhoea soon after feeding or by faecal discharge in or around the tube but is often asymptomatic and undiagnosed until the tube is replaced This type of injury mostly does not lead to early peritonitis.44 46 Minimising the risk for this complication can be achieved by paying careful attention to safe procedure technique during placement.14
Placement of a PEG through the colon. This patient was admitted to our hospital with anaemia. Faecal blood loss resulted in a diagnostic colonoscopy. The PEG was removed without any further complication. PEG, percutaneous endoscopic gastrostomy.
Prevention
Be extra cautious in patients with previous upper abdominal surgery or pathology.
Position the patient in a reverse or anti-Trendelenburg position during the procedure.
Choose an appropriate gastrostomy tube site.
Provide full gastric insufflation to displace the colon during endoscopy.
Check for proper transillumination through the abdominal wall of the light source at the distal tip of the gastroscope.
Ensure endoscopic visible imprint of a finger palpation on the skin.
Establish a ‘safe tract’ technique: endoscopic visualisation of a needle (eg, syringe filled with saline or local anaesthetic) and simultaneous return of air into the syringe. Return of fluid or gas into the syringe prior to endoscopic visualisation of the needle in the gastric lumen suggests entry into bowel interposed between the abdominal wall.
Management options
Conservatively: pull the PEG and apply a dressing over the tract.
Occasionally surgery is required to address colocutaneous fistulas.
Gastrocutaneous fistulae
Description
Gastrocutaneous fistulae (GCF) are a difficult to manage complication post gastrostomy removal. The estimated incidence of chronic GCF is about 4.5% but can be much higher particularly in children. In a retrospective study, 44 children (28 with a PEG and 16 with a surgical gastrostomy), 25% developed a GCF after removal.47 This high incidence was confirmed in a systematic review and meta-analysis.48 Fistula formation due to epithelialisation of the fistula tract has been shown to be associated with the duration of gastrostomy use (>6 months). The majority of gastrostomy sites close spontaneously after 1–3 months but if not, medical treatment to reduce gastric discharge and nutritional support to optimise wound healing and nutritional status, should be applied. Refractory cases can cause considerable morbidity including cutaneous injury, risk of infection, dehydration and requirement for frequent dressing and stoma bags. These patients are candidates for more invasive management usually combining endoscopic procedures with de-epithelialisation.
Prevention
Be aware (certainly in children) that a GCF can occur after the tube has been in place long term (>6 months).
Management options
Conservative (medical and/or nutritional)21 49–52:
Depending on gastric discharge, treat conservatively by applying a dressing over the tract site (first allowing stomal tract to completely heal for at least 1 month).
Consider the use of a stoma bag over the fistulous site and measure output.
Use proton pump inhibitors (40–80 mg daily).
Consider prokinetics (metoclopromide 10 mg three times a day).
Consider to add a somatostatin analogue.
Maintain medical therapy up to 8 weeks.
Keep the patient nil-by-mouth.
Consider postpyloric feeding through a nasojejunal feeding tube.
Consider total parenteral nutrition through a midline or central venous catheter.
Endoscopic or surgical options49–51 53–57:
Apply endoscopic clip closure (using haemoclips, bear claw clips or clips with radial design (over-the-scope-Padlock clip).
Apply endoscopic clip closure combined with de-epithelialisation (eg, with electrocautery, biopsy forceps or argon plasma coagulation).
Apply endoscopically assisted suturing.
Apply silver nitrate in the tract and external orifice, and closure of the internal orifice with clips.
Instillate under endoscopic guidance fibrin glue via the external opening through the whole fistulous tract.
Apply surgery with laparotomy and excision of the fistula tract.
PEG tract tumour seeding
Description
A rare complication after PEG placement is tumour growth (from the initial tumour) at the PEG insertion site in patients with oropharyngeal and oesophageal malignancies (see figure 8). A recent systematic review revealed 121 case reports.58 Generally it is believed that tumour seeding occurs during the ‘pull’ or ‘push’ method when the tube is in direct contact with the tumour during the procedure.59 However, some authors consider haematogenous or lymphatic spread of the tumour cells as the main mechanism of metastasis in some instances.8 In a large study with 777 patients analysed, a total of five patients with head and neck malignancy were identified with abdominal wall metastasis with an overall incidence of 0.64% over an average follow-up of 27.55 months.60 In a smaller study in patients with mixed oesophageal and oropharyngeal tumours malignant cells (on brushings) were present in 9.4 %, all of which were from oesophageal squamous cell carcinoma mainly in the older age group and higher tumour stages. Whether brushing as a surrogate endpoint is clinically relevant in predicting evolution to abdominal wall tumours is unclear.61 Besides pharyngoesophageal primary cancer, squamous cell histology, less well-differentiated cancer, large size and advanced cancer stage were reported as additional risk factors with poor mean survival after diagnosis.59 62 63
Abdominal wall metastasis at the gastrostomy insertion site which occurred a few months after PEG removal. PEG, percutaneous endoscopic gastrostomy.
Prevention
Avoid the ‘pull’ technique in high-risk patients and use instead direct access through the abdominal wall, using an introducer technique.
Management options
Consider aggressive therapy, if detected early, which may eventually provide a chance of cure but patients with abdominal wall metastases often have a poor prognosis.
Liver injury
Description
Just as with colon interposition a part of the liver can interpose between the abdominal wall and the stomach (see figure 9). Fistula formation can occur when a PEG first passes the left lateral segment of the liver which lies in close proximity to the stomach. All the blood leaving the stomach and intestines passes through the liver so injuries might be associated with bleeding, either during placement or at the time of removal but in many cases it will be completely asymptomatic and diagnosed in delayed fashion.64
Interposition of the liver between the abdominal wall and the stomach with the PEG passing through the liver. PEG, percutaneous endoscopic gastrostomy.
Prevention
Identify the caudal edge of the liver using percussion. The contrast between a dull and hollow sound allows for determination of the lower edge of the liver. This could be routinely done before PEG placement.
Management options
Removal can be done endoscopically or surgically.
Observe for signs of bleeding after removal.
An overview of the most relevant discussed preventive measures are summarised in table 1.
Overview of major post procedural percutaneous endoscopic gastrostomy complications and their prevention
Conclusion
Complications of gastrostomy placement may be minor or major. Fortunately the majority of gastrostomy placement complications are minor but nevertheless often affecting quality of life. Major complications are rare but prevention and early recognition are important. In this regard, the presence of a multidisciplinary nutritional support team can play a very important role in decreasing morbidity and mortality.14 15 30 65 66 This review was written from a clinical viewpoint and focused on evidence-based recommendations to prevent and manage major adverse events.
Data availability statement
No data are available. Data from illustrations are deidentified.
References
Footnotes
Contributors Both authors (KB and ID) contributed to the design of the study, KB drafted the manuscript and KB and ID critically revised the manuscript for important intellectual content and final approval.
Funding The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests None declared.
Provenance and peer review Not commissioned; externally peer reviewed.
Supplemental material This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.