Article Text
Abstract
Introduction and purpose Chronic pancreatitis (CP) involves progressive inflammatory changes to the pancreas and can lead to permanent structural damage and impairment of both endocrine and exocrine functions. Current reports highlight a rise in the incidence and prevalence of CP. However, there is limited data currently available concerning patients with CP undergoing exercise therapy (ET). We aim to prospectively examine the influence of ET on sarcopenia in patients with CP.
Methods and analysis A detailed evaluation of the nutritional condition and the daily physical activities of each participant will be conducted prior to entering the study. Our patients will be randomly allocated to either: (1) the ET group or (2) the control group. In the ET group, our patients with CP will receive nutritional guidance once a month. The patients with CP will also be instructed to perform exercises with > 3 metabolic equivalents (mets; energy consumption in physical activities/resting metabolic rate) for 60 min/day and to perform exercises >23 mets/week. The primary end point will be an improvement in sarcopenia, defined as an increase in muscle mass and muscle strength, at 3 months postrandomisation. A comparison of the amelioration of sarcopenia in the two groups will be undertaken.
Ethics and dissemination The Institutional Review Board at Hyogo College of Medicine approved this study protocol (approval no. 2766). Final data will be publicly announced. A report releasing the study results will be submitted for publication to an appropriate journal.
Trial registration number UMIN000029263; Pre-results. No patient is registered at the submission of our manuscript.
- abdominal pain
- chronic pancreatitis
- nutrition
- nutritional status
This is an Open Access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Statistics from Altmetric.com
Summary box
To the best of our knowledge, the current study is the first prospective interventional study (randomised trial) to examine the influence of exercise therapy in patients with chronic pancreatitis on sarcopenia.
Our study results will be limited to a Japanese population, and further validation studies on other ethnic backgrounds will be required.
Introduction
Chronic pancreatitis (CP) involves progressive inflammatory changes of the pancreas.1–5 In general, CP first presents with repeated upper abdominal and back pain.1–5 CP can lead to permanent structural damage, which can result in the impairment of both endocrine and exocrine functions.6 7 CP has a detrimental effect on patient quality of life.8 9 Current reports highlight a rise in the incidence and prevalence of CP, and CP is reported to be more common among male individuals.6 7 In Japan, CP is classified into three clinical phases: compensated, transitional and decompensated, depending on the clinical stage.1 Exocrine pancreatic impairment and inadequate glucose tolerance can occur as CP progresses1 2 10 11; therefore, to ameliorate the prognosis in patients with CP, pharmacological and nutritional interventions should be based on the clinical stage of the disease.6 10 11 Regular physical activity favourably influences the risk for disease onset and the progression of several chronic diseases.12–17 However, investigations on the influence of exercise therapy (ET) on CP are relatively scarce.
The loss in muscle mass and muscle strength associated with ageing is termed primary sarcopenia.18–20 Secondary sarcopenia is defined as the loss of muscle mass and muscle strength that accompanies underlying diseases such as chronic kidney disease, chronic liver diseases, advanced malignancies and malnutrition (inadequate protein or caloric intake).18 19 21 22 Patients with CP have more pronounced levels of malnutrition.1 23 24 Sarcopenia and serum albumin levels are reported to be closely associated with pancreatic exocrine insufficiency in patients with pancreatic disease.25
As described above, ET conveys multiple health benefits both in healthy individuals and for several chronic diseases.12–17 26–28 Despite these clinical benefits, there is limited data available regarding patients with CP undergoing ET. Therefore, there is a need to investigate this issue. In this study, we aim to prospectively examine the effect of ET on sarcopenia in patients with CP.
Patient eligibility criteria
From a clinical practice perspective, it should be emphasised that, in malnourished patients with CP, ET may be accompanied by increased health risks, as ET may accelerate further protein catabolism and muscle mass decrease.1 24 29 At the time of patient registration, a precise evaluation of the nutritional condition and the daily physical activities performed will be conducted individually for each participant.
Inclusion criteria
Both sexes.
Patients with CP aged 20 years and over. A diagnosis of CP will be based on the current Japanese guidelines.1
Aetiologies for CP are not limited.
Exclusion criteria
Patients with severe depression or psychiatric disorder.
Patients with acute pancreatitis or those with such severe CP that participation in this study is anticipated to be difficult.
Patients with severe underlying diseases, such as advanced malignancies (including pancreatic cancer), severe infectious diseases, severe chronic heart failure and respiratory disorders.
Pregnant or lactating female patients.
Patients who may be at a risk of falls.
Patients considered unsuitable for the study because of the inability to participate in ET.
Patients with a previous history of surgery or endoscopic therapy for pancreatic diseases.
Patients with severe pancreatic ascites or pseudocysts.
Study protocol
Study design: non-double-blind randomised controlled trial
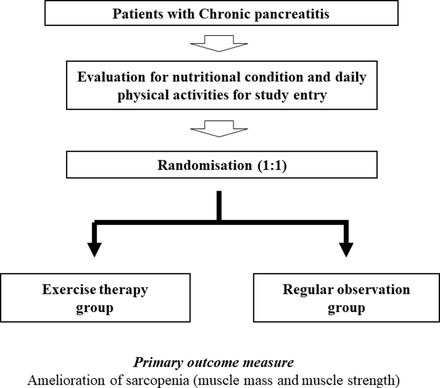
Our study participants are patients with CP. All CP stages (compensated, transitional and decompensated) will be included. Pharmacotherapy and diet therapy for CP will be permitted. Study participants will be randomly allocated into two groups: (1) the ET group or (2) the control group (regular observation group) (figure 1).
Study design.
Exercise therapy
In the ET group, nutritional advice will be provided for each participant once a month at the outpatient nutritional guidance clinic. Participants will also be instructed to perform exercises with > 3 metabolic equivalents (mets; energy consumption in physical activities/resting metabolic rate) for 60 min/day and to perform exercises >23 mets/week.12–17 In the ET group, physical activities equal to or higher than walking for 60 min/day will be strongly recommended for each study participant. In both groups, pharmacotherapy, such as protease inhibitors and pancreatic enzyme replacement therapy, and diet therapy, such as a fat restriction diet, in each underlying pancreatic disease will be permitted and we will ask all study participants to self-declare their daily amount of exercise.1 Direct monitoring of exercise will not be undertaken.
Primary end points
Sarcopenia improvement
Sarcopenia will be defined based on the current Asian guidelines.20 Muscle mass, using bioimpedance analysis (BIA) and analysis of muscle strength (hand-grip strength) for the evaluation of sarcopenia, will be calculated once each month. Amelioration of sarcopenia at 3 months postrandomisation will be the primary end point. We will prospectively compare the amelioration of sarcopenia within the two groups.
Secondary end points (examination for study)
Changes over time in baseline characteristics
We will assess changes over time to the following baseline characteristics: body weight, body mass index, white blood cell count, platelet count, serum albumin level, aspartate aminotransferase, alanine aminotransferase, total cholesterol, triglyceride, low-density lipoprotein, high-density lipoprotein, fasting blood glucose, haemoglobin A1c, homeostasis model assessment of insulin resistance and the N-benzoyl-L-tyrosyl-p-aminobenzoic acid test.1
Follow-up and standard of care
During the observation period and after completion of the study, all participants will be seen in clinic every 4 weeks to address complications from CP and other comorbidities. Compliance with pharmacotherapy will be particularly emphasised. Regular laboratory tests (haematology, biochemistry and coagulation) will be requisite at the study entry and at the completion of study, and on an as-required basis.
Case registration period
From October 2017 to March 2021.
Data collection
A study assistant will gather data elements from the patient medical records, including:
Baseline data:
Sex and age;
Height and body weight;
Vital signs;
History of alcohol consumption and history of smoking;
Aetiology of underlying pancreatic disease;
Clinical stage of CP (compensated, transitional or decompensated);
Previous treatment and medication;
Comorbid conditions;
Baseline laboratory tests;
Presence or absence of ascites on radiologic findings.
Statistical methods
Descriptive statistics
Data will be entered using JMP software (SAS Institute, Cary, North Carolina, USA), and all data will be checked to confirm consistency. Data at each time point will be compared. Quantitative parameters will be compared using a paired or an unpaired t-test. Categorical parameters will be compared using Pearson’s χ2 test or Fisher’s exact test, as applicable.
Sample size estimation
According to our previous BIA results, supposing that the α error (type 1 error) is 0.05, the detection power (β) is 0.8, the difference in the two groups to be detected and measured using BIA is 10, and the SD of outcome is 10, the number of required participants in both groups will be 17 (total of 34 participants) in order to randomly allocate one to one.30 31 Randomisation will be performed using a dedicated computer. We anticipate that a number of participants may drop out of the study; therefore, a total of 40 participants will be required to confirm our hypothesis.
Discussion
The population in Japan has obtained the longest life expectancy in the world.16 Japan is an ageing country, and the clinical importance of ET has recently gained considerable attention due to the multiple health benefits of ET.12–17 26–28
Patients with CP potentially have a more pronounced level of malnutrition.1 23 24 Therefore, we consider CP to be closely associated with sarcopenia. However, there are no existing data regarding the benefits of ET for patients with CP on sarcopenia. To the best of our knowledge, this is the first prospective interventional study that will objectively evaluate the influence of ET on sarcopenia in patients with CP. From a clinical practice perspective, we highlight the potential safety risks of ET in malnourished patients with CP, because ET may risk promoting further protein catabolism and muscle mass loss. An adequate nutritional evaluation will be required prior to starting ET in our study cohort, and this study will be performed with full care.
One of the major strengths of our study is that this will be a randomised controlled trial (RCT). One study limitation is that this study will be based solely on a Japanese population. Additional investigations in different ethnic populations are required to further verify the efficacy of ET in sarcopenia and to extrapolate our findings to other ethnicities. However, if the clinical efficacy of ET for sarcopenia in CP participants is confirmed in this RCT, the information we provide may be beneficial to clinicians.
Ethics and dissemination
Research ethics approval
The study protocol, the informed consent form and other submitted documents have been reviewed and approved. The trial registration number is UMIN000029263 (https://upload.umin.ac.jp/); preresults. No patient is registered at the submission of our manuscript.
Confidentiality
On patient recruitment, the study assistant will attribute a unique scrambled identification (ID) number to each study participant. Only the ID number will be used to identify the participants. Data sheets and any printouts of electronic files will be stored in a locked filing cabinet in a secure office at the Department of Hepatobiliary and Pancreatic disease, Department of Internal Medicine, Hyogo College of Medicine, Hyogo, Japan, with restricted access.
Dissemination policy
Final data will be made public irrespective of the study results. A report releasing the study results will be submitted for publication to an appropriate journal after data collection has been completed.
References
Footnotes
Contributors KY designed the study and will write the initial draft of the manuscript. HN and HE will contribute to the analysis and interpretation of the data, and will assist in the preparation of the manuscript. The other remaining authors will contribute to data collection and interpretation, and will critically review the manuscript.
Competing interests None declared.
Ethics approval This study has received approval from the Institutional Review Board at Hyogo College of Medicine (approval no. 2766).
Provenance and peer review Not commissioned; externally peer reviewed.