Article Text
Abstract
Background Three large randomised trials have shown that screening for colorectal cancer (CRC) using the faecal occult blood test (FOBt) can reduce the mortality from this disease. The largest of these trials, conducted in Nottingham since 1981, randomised 152 850 individuals between the ages of 45 and 74 years to an intervention arm receiving biennial Haemoccult (FOB) test kit or to a control arm. In 2006, the National Bowel Cancer Screening Programme was launched in England using the FOBt, with the expectation that it will reduce CRC mortality.
Aims To compare the CRC mortality and incidence in the intervention arm with the control arm after long-term follow-up.
Methods The 152 850 randomised individuals were followed up through local health records and central flagging (Office for National Statistics).
Results At a median follow-up of 19.5 years there was a 13% reduction in CRC mortality (95% CI 3% to 22%) in the intervention arm despite an uptake at first invitation of approximately 57%. The CRC mortality reduction in those accepting the first screening test, adjusted for the rate of non-compliers, was 18%. There was no significant difference in mortality from causes other than CRC between the intervention and control arms. Despite removing 615 adenomas >10 mm in size from the intervention arm, there was no significant difference in CRC incidence between the two arms.
Conclusions Although the reduction in CRC mortality was sustained, further follow-up of the screened population has not shown a significant reduction in the CRC incidence. Moreover, despite the removal of many large adenomas there was no reduction in the incidence of invasive cancer which was independent of sex and site of the tumour.
- Faecal occult blood testing
- colorectal cancer
- screening
- adenoma
- colonoscopy
- abdominal surgery
- cancer susceptibility
- colon carcinogenesis
- clinical trials
- colorectal cancer screening
Statistics from Altmetric.com
- Faecal occult blood testing
- colorectal cancer
- screening
- adenoma
- colonoscopy
- abdominal surgery
- cancer susceptibility
- colon carcinogenesis
- clinical trials
- colorectal cancer screening
Significance of this study
What is already known about this subject?
Faecal occult blood (FOB) testing is widely used in screening the population for colorectal cancer (CRC).
FOB testing leads to a reduction in disease-specific mortality.
Compliance with FOB test screening, in general, is around 60%.
What are the new findings?
Long-term follow-up of a large randomised trial of FOB test screening with a total of 150 000 subjects.
Sustained reduction in CRC mortality in subjects invited for screening when followed up for 20 years.
No significant reduction in CRC incidence, which has implications for the existing National Bowel Cancer Screening Programme.
How might it impact on clinical practice in the foreseeable future?
Screening by the FOB test is worthwhile because it reduces mortality from a common cancer.
The National Bowel Cancer Screening Programme can expect to deliver mortality reduction from CRC.
CRC screening is likely to continue to develop using newer tests such as immunological FOB testing.
Introduction
Colorectal cancer (CRC) is the second most common cause of death from malignant disease in the UK. Three randomised trials1–3 have shown a reduction in the risk of death from CRC as a result of faecal occult blood test (FOBt) screening and a meta-analysis including a fourth unpublished study using Haemoccult found a 16% reduction in CRC mortality (95% CI 10% to 22%) in the populations offered screening.4 On the basis of the results of these randomised trials, the National Bowel Cancer Screening Programme (NBCSP) was launched in England in 2006. Men and women aged 60–69 years are offered FOBt screening every 2 years and the upper age limit is currently being extended to 74 years.
While there is accumulating evidence to show that screening for CRC is of benefit, there are concerns about the potential morbidity resulting from such a screening programme, specifically complications arising from colonoscopy, such as perforation of the colon, and the psychological harm arising from a false positive test. Ideally, screening for CRC should show a reduction in CRC mortality and a reduction in the incidence of CRC as a consequence of removing large adenomas in the screened population. However, the long duration of the adenoma-carcinoma sequence (estimated to be 10–15 years on average) and the fact that large adenomas bleed intermittently (reducing the sensitivity of FOBt) means that prolonged follow-up may be necessary to show any significant reduction in incidence of CRC.
This paper presents the long-term follow-up of the randomised trial of FOB screening that started in Nottingham in 1981, and that has now been followed for a median of 19.5 years. Results from this trial at a median of 11 years follow-up showed a 13% reduction in CRC mortality.3 In the Minnesota randomised trial, 18-year follow-up of biennial screening had shown significant reductions in both mortality from and incidence of CRC.2 The present analysis was conducted to determine the long-term effects of screening on CRC mortality and to determine whether a reduction in incidence could be observed.
Methods
Between February 1981 and January 1991, 152 850 subjects aged 45–74 years living in the Nottingham area were randomly allocated to an intervention or control arm.
The subjects in the intervention arm were offered biennial screening by FOBt, the majority of individuals being offered between three and five tests according to their date of entry. Randomisation was by household, but over half the subjects were in single-person households. Subjects in the control arm were not told about the study and received no intervention but usual medical care. The methodology of the trial is described in more detail elsewhere.5 It is now over 18 years since the last subjects were enrolled and 15 years since screening ceased (median follow-up is 19.5 years). The whole population has been followed up through local hospital records and flagging at the NHS central register (Office of National Statistics) to determine the subsequent incidence of CRC and mortality from all causes. Flagging of individuals in the trial facilitated identification of non-screen-detected cancers presenting to other hospitals in the UK. Adenomas removed from the control arm and those in the intervention arm not screen-detected were found as a consequence of investigation (usually by colonoscopy or in the early days of the study by barium enema) and subsequent endoscopic follow-up of symptomatic presentation. They were identified through histopathology records of the hospitals in Nottingham and the surrounding area.
Structured case note reviews of certified deaths due to CRC and deaths in registered CRC cases were carried out in order to obtain more reliable information on the cause of death. CRC mortality rates were calculated from the underlying cause as stated on the death certificate and the verified cause of death after case note review.6 The verification process was blind to whether the patient was in the control or intervention arm, although in around 5% of cases it was apparent from the notes that the patient had been screened.
Person-years of follow-up were calculated from date of entry into the study to death or 30th June 2009, whichever was earlier. Cumulative mortality and incidence rates were calculated by dividing the total number of deaths from, or cases of, CRC by the total number of subjects randomised to that arm, at each year since randomisation. However, the plots of these rates reflect the decreasing proportion of subjects followed up with increasing time since randomisation (due to the 10-year period over which randomisation was carried out and death or loss of participants due to migration). In addition, Nelson–Aalen estimates of cumulative mortality and incidence were calculated from the number of deaths (or cases) in each year since randomisation divided by the number of person-years observed during that year and summing these individual rates.
A standard ‘intention to treat’ analysis was performed to calculate estimates of relative incidence and mortality. Poisson regression, offset by the natural logarithm of person-years of observation, was used to calculate incidence and mortality rate ratios relative to the control arm and also provide 95% CIs for these estimates. The variation in these rate ratios with age and sex was also investigated using Poisson regression. Poisson models were compared by the likelihood ratio test. Proportions were compared using the χ2 test.
Screening in the trial ended in February 1995, and the reduction in CRC mortality will be increasingly diluted by deaths occurring in both trial arms in cases diagnosed after the end of fieldwork, which in the intervention arm could not have benefited from screening. An analysis has therefore also been carried out restricted to deaths in cases diagnosed up to 12 years from date of entry, at which point the CRC incidence in the two arms was approximately equal, thus ensuring that where deaths occur in the intervention arm in cases for which diagnosis is advanced by screening, the equivalent deaths are included in the control arm.
Analysis was also carried out on a ‘per protocol’ basis modified to allow for differences in underlying rates in the acceptors and non-acceptors of screening, in order to produce an estimate of relative risk (RR) of CRC mortality in those accepting the first screening test relative to the control arm.7
Cause-specific mortality has been investigated in subjects in the control and intervention arms, the latter subdivided according to whether they had accepted at least one test and the results of this analysis were reported previously.3
Results
Of the 152 850 individuals recruited, 76 466 were randomised to the intervention arm and 76 384 to the control arm; 875 (0.6%) could not be traced by the Office for National Statistics or had emigrated and were therefore excluded from the analysis. Of the remaining 151 975, 76 056 were in the intervention arm and 75 919 were in the control arm. Median follow-up was 19.5 years (range 0.0–28.4 years, IQR 10.2).
Detection rates of CRC were 2.1 per 1000 subjects at first screen and 1.5 per 100 at subsequent routine screens; the adenoma detection rates were 8.4 and 4.4 per 1000, respectively (2.9 per 1000 ≥20 mm or 7.2 ≥10 mm).
A total of 2279 CRCs occurred in the intervention arm compared with 2354 in the control arm (table 1). Of those in the intervention arm 236 were screen-detected, 1037 presented as interval cancers (between screening rounds) and 47 were detected as a consequence of follow-up protocols for subjects with screen-detected adenomas. Screen-detected cancers were more likely to be a Dukes' stage A or B tumour (71%, 168/236) compared with those in the control arm (42%, 993/2354). The distribution of cancer stage in the intervention and control arms has been documented in previous papers.5
Stage of colorectal cancer in intervention and control arms
During the period of the trial and subsequent follow-up 40 681/76 056 (53.5%) individuals in the intervention arm died (table 2). In the same period 40 550/75 919 (53.4%) individuals in the control arm died. Deaths from verified CRC showed a statistically significant reduction in the intervention arm (RR 0.91, 95% CI 0.84 to 0.98). The reduction in deaths from certified CRC was similar (RR 0.91, 95% CI 0.84 to 0.99). There was no significant difference between the reduction in men and women or in those under 60 years and those 60 years and over at randomisation or according to tumour site (table 3).
Colorectal cancer incidence and mortality rates and ratios in intervention and control arms
Verified colorectal cancer mortality by age, sex and site of cancer
The CRC mortality reduction in those accepting the first screening test, adjusted for the rate of non-compliers, was 18% (RR 0.82, 95% CI 0.70 to 0.95). The overall reduction of 9% in CRC mortality observed here will have been diluted by deaths occurring in cases diagnosed after the end of the fieldwork, which could not have benefited from early diagnosis by screening. If the mortality analysis was restricted to cases diagnosed within 12 years from date of entry, the reduction in the intervention arm was 12% (RR 0.88, 95% CI 0.79 to 0.98).
The absolute reduction in mortality from CRC was 1.66 per 1000 persons, giving a number of 602 persons (95% CI 339 to 2648) who need to be invited for screening for an average of 6 years to prevent one death over 20 years.
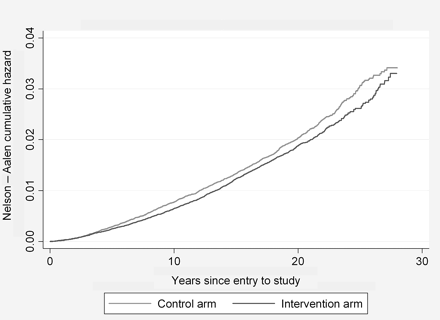
Cumulative incidence at the end of the follow-up period was not significantly reduced in the intervention arm compared with the control arm (RR 0.97 (CI 0.91 to 1.03) p=0.28; table 2) (figure 1).
Cumulative incidence of colorectal cancer.
A total of 2291 adenomas were removed from 1991 subjects in the intervention arm and 1484 from 1343 subjects in the control arm (table 4). Of the adenomas removed from patients in the intervention arm, 615 were screen-detected adenomas of ≥10 mm size removed at colonoscopy. In total (including non-responders, interval adenomas and those detected on polyp follow-up) 1423 (62%) adenomas in the intervention arm were ≥10 mm in diameter and 461 (20%) were ≥20 mm were detected. During the same time period 883 individuals in the control arm had adenomas ≥10 mm removed (figure 2).
Size of a patient's largest adenoma in intervention and control arms
Cumulative mortality from colorectal cancer.
The number needed to be screened (as opposed to invited) to save one life was 300 (adjusted for non-attender bias).
Discussion
The results of the Nottingham trial at a median follow-up of 19.5 years continue to show a reduction in the CRC mortality in the intervention arm, although the reduction in cumulative mortality is diluted by deaths occurring in cases diagnosed after the end of screening. We have previously reported a mortality reduction of 15% at a median follow-up of 7.8 years3 and a 13% reduction at 11 years.(Scholefield & Moss 2002) In the current analysis, restriction to deaths in cases diagnosed within 12 years of entry gives a similar reduction to the latter.
There was no significant reduction in CRC incidence even after a median of 19.5 years of follow-up, which would provide over 90% power to detect a 10% reduction in the intervention arm. The removal of 540 (1423–883) additional adenomas of ≥10 mm in size in the intervention arm had only a small effect on the incidence of CRC. This can be interpreted as further evidence that the adenoma-carcinoma sequence is a slow process taking in excess of 15 years even for large adenomas. Removing small or medium-sized adenomas also appears to have a small impact on a patient's cancer risk. Both these observations would suggest that the intervals between endoscopic surveillance of patients with colonic adenomas could be increased further or discontinued for low risk adenomas (indeed, the policy in the NBCSP is that patients with low-risk adenomas do not receive follow-up.
Although the Nottingham trial was designed to show a reduction in CRC mortality, it seems logical to believe that removal of adenomas in the screened population would lead to a reduction in incidence of CRC. However, this was not observed despite a prolonged period of follow-up. Only 615 large adenomas (≥10 mm) were detected by screening and removed from the intervention arm of over 76 000 people. This may represent too small a number to cause a significant reduction in incidence. In addition, 883 adenomas ≥10 mm were removed from subjects in the control arm, who presented symptomatically over the 20-year period of the study and this may have diluted the effect of removing adenomas from the screened population.
The Minnesota trial showed a reduction in both mortality from and incidence of CRC at 18 years of follow-up. The arm of this trial that used biennial screening had a compliance rate of 78% and 28% of the screened subjects had a colonoscopy, equivalent to approximately 22% of the population invited.8 Rehydration of the majority of FOBts and classification of any test with at least one positive square as positive led to a considerably higher positive rate, and the positive predictive value was lower than in the Nottingham trial.9 In the biennial arm of the Minnesota trial a total of approximately 444 adenomas >10 mm were detected by screening, in a population of 15 500. Evidence of a divergence in the incidence of CRC in the Minnesota trial began to emerge at around 13 years of median follow-up.8
In comparison, the proportion of screened subjects in the Nottingham trial who had a colonoscopy was around 5%.
Thus it is likely that a higher rate of detection/removal of large adenomas than was achieved in the Nottingham trial is necessary to have an effect on subsequent cancer incidence. Further follow-up is unlikely to alter the incidence of CRC in this trial as over 50% of the trial population have now died.
There were seven complications of colonoscopy consequent upon positive FOB testing (7/1474 initial screening colonoscopies), six of which required surgical intervention (five perforations and one bleed), but no mortality was caused by colonoscopy.10 There is no evidence that screening for CRC causes any lasting psychological harm to participants, although a positive FOBt does cause a transient rise in anxiety scores.11
The distribution of cancers by site and stage has been extensively described in previous reports.5
The occurrence of over 1000 interval cancers in the intervention arm reflects the limited sensitivity of FOB screening, which we have previously estimated as 54%.12 Only 27/2279 (1.2%) cancers occurred in subjects following a positive FOBt with negative investigations. There were seven cancers missed on colonoscopy or barium enema undertaken after failed colonoscopy.5
Compliance in the Nottingham trial was only 57% and results from the English FOBt pilot suggest compliance in the NBCSP may be similar, at least initially. Complete roll out of the programme was achieved in 2010, but it will be a further 8–10 years before the full impact on disease-specific mortality is likely to be observed.5
All analyses are based on data up to 30 June 2009.
References
Footnotes
Funding This study was supported by the Medical Research Council with several project grants from 1987 to 2009 for data collection and trial administration.
Competing interests None.
Patient consent This trial was conceived in 1985, when permission from participants in a trial was not required. Since, it would not be possible to go back over 20 years later and seek permission to report the results of the trial, particularly as over half of those individuals randomised to participate are now deceased, no consent was obtained.
Ethics approval This study was approved by the Nottingham Family Practitioner Committee and BMA Medical Ethics committee in 1985. The requirements for trial registration did not exist at the start of this trial. Similarly in 1985 when this trial was being developed there were no formal ethics committees in each region, hence ethical permission was sought from the local Family Practitioner Committee as these patients were identified in general practice and the trial was approved by the BMA ethics committee as these were deemed the most appropriate bodies at the time.
Provenance and peer review Not commissioned; externally peer reviewed.
Data sharing statement The data in this paper can be obtained from the corresponding author.