Article Text
Abstract
Background In patients with chronic hepatitis C (CHC) cirrhosis, imaging for hepatocellular carcinoma (HCC) is recommended every 6 months to maximise eligibility for curative treatment. The aim was to determine the adherence rate and outcomes among patients with CHC cirrhosis and whether the adherence rate has improved over time.
Methods Retrospective cohort study of patients with CHC cirrhosis (n=2366) monitored for ≥1 year at Stanford University Medical Center between January 2001 and August 2015.
Results Overall demographics: mean age 54; 62.3% men; 48.3% Caucasian. 24.4% adherent to imaging every 6 months per European Association for the Study of the Liver 2000 and American Association for the Study of Liver Diseases (AASLD) 2011 criteria and 44% at least every 12 months per AASLD 2005 criteria. No significant change in adherence before and after 2011. Predictors of multivariable analysis of adherence were age >54 (OR 1.74, p<0.0001), Asian ethnicity (OR 2.23, p<0.0001), liver decompensation (OR 2.40, p<0.0001) and having ≥2 clinical visits per year (OR 1.33, p=0.01). During follow-up, 9.6% were diagnosed with HCC. Adherent patients were more likely to have smaller tumours (2.3 vs 3.3 cm, p=0.0020), be within the Milan criteria for liver transplants (73.2% vs 54.8%, p=0.006) and receive curative HCC treatment (43.6% vs 24.0%, p=0.005). On multivariable analysis, curative treatment (HR 0.32, p=0.001) and every 6-month imaging (HR 0.34, p=0.005), but not every 6–12 month imaging, were associated with reduced risk of mortality.
Conclusions Adherence to HCC surveillance continues to be poor. Adherent patients with HCC were more likely to undergo curative treatment and have better survival. Research understanding barriers to surveillance is needed.
- screening
- hepatocellular carcinoma
- liver cirrhosis
- hepatitis C
This is an Open Access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Statistics from Altmetric.com
Summary box
What is already known about this subject?
Major liver disease societies recommend surveillance for hepatocellular carcinoma (HCC) in high-risk groups to maximise eligibility for curative treatment.
The American Association for the Study of Liver Diseases (AASLD) 2011 and European Association for the Study of the Liver (EASL) 2000 guidelines recommend HCC surveillance every 6 months.
The AASLD 2005 guideline recommends surveillance every 12 months.
What are the new findings?
In a large, real-life cohort of patients with chronic hepatitis C cirrhosis, only 24% underwent HCC surveillance every 6 months and only 44% had surveillance at least every 12 months.
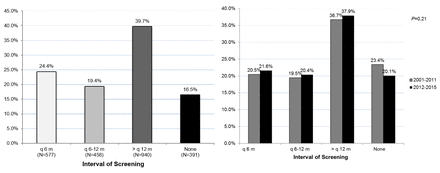
Adherence rates remained poor over the 16 years of the study: the every 6 month surveillance rate was 20.5% before 2011 and 21.6% after 2011 (p=0.21).
The 5-year cumulative survival was 54.7% for the adherent (every 6-month imaging) group, compared with 6.5% for the non-adherent group (p<0.00001). Although every 6-month imaging reduced mortality by 66%, every 6–12 month imaging did not.
Besides age >54, Asian ethnicity and decompensation, more frequent clinic visits was associated with a 33% increase in every 6-month imaging.
How might it impact on clinical practice in the foreseeable future?
Further research to understand barriers to surveillance is needed, but our study suggests that just one additional clinic visit per year increases the likelihood of undergoing surveillance.
Introduction
Chronic hepatitis C (CHC) is a major burden, with an estimated 71.1 million infections worldwide. In 2015, the USA had the sixth highest number of cases with approximately 3 million infections.1 The prevalence of cirrhosis and hepatocellular carcinoma (HCC) in patients with CHC has been increasing, and CHC is now the top cause of end-stage liver disease, HCC and liver-related death in the western world.2–6
Approximately 10%–20% of patients with CHC develop cirrhosis. Over time, compensated cirrhosis can advance to decompensated cirrhosis, which is associated with a 15%–20% risk of death in the year following the first episode of decompensation.2 In the USA, CHC-associated mortality has been increasing and the number of associated deaths has now surpassed that of 60 other nationally notifiable infectious conditions, including HIV infection.2 A common cause of liver-related death among patients with CHC is HCC, usually seen in those who have also developed cirrhosis.7
As such, HCC is now the sixth most common cancer, and the second leading cause of cancer mortality worldwide.8 In the USA, HCC incidence has increased from 1.51 cases per 100 000 in 1973 to 6.20 cases per 100 000 in 2011.9 Survival is generally very poor, except in patients who receive curative treatment (liver transplantation, surgical resection or radiofrequency ablation).10 11 Eligibility for curative treatment diminishes with more advanced disease; therefore, major liver disease societies recommend surveillance for HCC in high-risk groups, such as patients with cirrhosis of any aetiology.12–14
The first guideline addressing HCC surveillance for patients with cirrhosis was the European Association for the Study of the Liver (EASL) 2000 guideline, which recommended ultrasound every 6 months for patients with cirrhosis of any cause.12 The American Association for the Study of Liver Diseases (AASLD) first addressed this issue in their 2005 guideline, recommending screening every 6–12 months for patients considered to be at high risk and when treatment would be cost-effective.13 After further study, the AASLD updated their guidelines in 2011, recommending ultrasound examination every 6 months for patients with cirrhosis, as a result of the potential for tumour volume doubling in <6 months.14 In fact, a recent study found that the median tumour volume doubling time for CHC-associated HCC was 137.2 days.15
Studies have found that HCC surveillance is associated with early HCC diagnosis, curative treatment and improved survival in patients with cirrhosis.16–19 However, the majority of these studies took place before the 2011 AASLD revision that recommended every 6-month imaging instead of 6–12 month imaging.20–25 Therefore, it is unclear if adherence in the USA improved over time and especially after the release of the 2011 AASLD guidelines. Furthermore, little is known about the predictors of adherence and the effects of adherence on tumour staging in patients with CHC cirrhosis.
Therefore, the goals of the current study were to examine adherence to the 2000 EASL and the 2011 AASLD surveillance guidelines for patients with CHC at a high risk for developing HCC, whether or not adherence has changed in recent years, and the effect of adherence on survival in a large, ethnically diverse population.
Methods
Study design and patient population
We performed a retrospective cohort study of consecutive patients with CHC cirrhosis monitored for at least a year at Stanford University Medical Center between January 2001 and August 2015. The start date of 2001 was chosen to allow for time for implementation of the 2000 EASL guidelines (surveillance every 6 months). The comparison group was based on the AASLD guidelines, which recommended every 6–12 months in 2005 but changed their recommendations to every 6 months in 2011. This time frame accounts for patients who underwent surveillance every 6 months and every 6–12 months. However, our primary analysis was based on every 6-month adherence.
Patients were identified via electronic query using International Classification of Diseases, Ninth Revision codes with subsequent data extraction in eligible patients via individual medical chart review. All CHC diagnoses were verified by a chart review based on evidence of positive hepatitis C antibody or positive hepatitis C virus RNA tests. All cirrhosis diagnoses were verified by a chart review based on histological diagnosis, in addition to mention of any of the following in radiology, laboratory records or physician’s notes: nodular contour, ascites, encephalopathy, splenomegaly, oesophageal varices, other varices or platelets <120 000/mL. HCC status was based on pathology or imaging using 2011 AASLD criteria.14
Patients were excluded if they were under 18 years of age, had <1 year of follow-up, had previously undergone liver transplantation, presented with HCC at baseline or were diagnosed with HCC within 6 months of the first visit. We included patients with Child-Pugh class C liver disease because liver transplantation is an option for these patients at the study site, and those with tumours within the Milan criteria are potential candidates for curative HCC treatment.
Follow-up time was defined as the period of time from initial presentation with CHC cirrhosis at the study centre to the most recent patient encounter, incident HCC diagnosis, liver transplantation for non-HCC indications or death. All-cause mortality data were obtained from a chart review and supplemented with a National Death Index search.
Definitions of surveillance and adherence for primary analysis
Surveillance was defined as undergoing a liver imaging test (ultrasound, CT or MRI). Although current guidelines recommend the use of ultrasound in high-risk patients, CT and MRI are also routinely used in clinical practice for HCC surveillance, especially when ultrasound imaging is suboptimal, as frequently is the case with cirrhotic livers.13 26
Adherence was defined as surveillance imaging every 6 months.
Non-adherence was defined as either undergoing surveillance less often than every 6 months but more often than every 12 months, less often than every 12 months or no surveillance at all.
Statistical analysis
Descriptive statistics were reported as proportions (%) for categorical variables and mean±SD or median (IQR) for continuous variables. Comparative analysis between groups was performed using the χ2 test for categorical variables. For continuous variables, the Student t-test was used to evaluate normally distributed continuous variables, and the Wilcoxon rank-sum test was used to evaluate continuous variables that were not normally distributed.
Stepwise multivariable logistic regression was used to estimate ORs and 95% CIs relating potential predictors to the outcome of optimal adherence. Survival was estimated by the Kaplan-Meier method, and survival curves were compared by the log-rank test. The Cox proportional hazards model was used to assess the overall survival for patients diagnosed with HCC. Time zero was HCC diagnosis and survival was until the event (all cause-mortality) occurred or patients were censored at the end of study follow-up. Patients were not censored if they received curative HCC treatment.
Statistical significance was defined as a two-tailed p value <0.05. Data analysis was performed using Stata V.14.2. This study was approved by the Institutional Review Board at Stanford University (Stanford, California, USA).
Results
From January 2001 through August 2015, through electronic query followed by an individual chart review using a case report form, we identified 2366 consecutive patients with CHC cirrhosis with 1 year of follow-up. Table 1 presents the cohort’s demographic and clinical characteristics. The overall mean age at baseline was 54 years (±10), with the majority being men (62.3%). Our cohort was 48.3% Caucasian, 21.9% Latino/Hispanic, 10.7% Asian, 5.6% African-American and 13.4% other/unknown. Almost half (45.6%) of the patients had an episode of decompensation at baseline, and 64.9% had a Model for End-Stage Liver Disease (MELD) score of at least 10. By Child-Pugh-Turcotte (CPT) classification, 42.0% were class A, 37.9% were class B and 20.2% were class C. The median number of clinical visits per year was 2.7 (range: 0.9–8). During the follow-up, 228 patients (9.6%) were diagnosed with HCC. 27.8% of patients died during a median follow-up of 35.6 (range: 22–62) months.
CHC cirrhotic patients’ demographic and baseline clinical characteristics overall and by adherent (every 6 months) versus non-adherent status
Rates of adherence to HCC surveillance
As shown in figure 1A, only 24.4% of patients underwent the recommended imaging every 6 months, while 19.4% had imaging every 6–12 months, 39.7% had imaging greater than every 12 months and 16.5% had no evidence of surveillance. Of note, rates of every 6-month imaging were similar before and after the 2011 AASLD guideline for HCC surveillance: 20.5% before 2011 and 21.6% after 2011 (p=0.21) (figure 1B).
(A) Adherence rates to HCC surveillance guidelines. (B) Adherence rates following the EASL 2000 HCC surveillance guidelines implementation compared with the AASLD 2011 change in HCC surveillance guidelines. AASLD, American Association for the Study of Liver Diseases; EASL, European Association for the Study of Liver Disease; HCC,hepatocellular carcinoma.
Patient characteristics by adherence status
Demographically, adherent patients compared with non-adherent patients were more likely to be older (mean: 56±9 vs 54±10, p<0.00001), Asian (17.3% vs 8.6%, p<0.0001), have decompensated cirrhosis (54.3% vs 42.8%, p<0.0001) and were less likely to drink alcohol (24.5% vs 36.2%, p=0.001) or smoke (11.8% vs 22.6%, p<0.0001) (table 1). Clinically, adherent patients were more likely to have a baseline MELD score ≥10 (71.3% vs 62.1%, p<0.0001) but were less likely to be within CPT class A (35.4% vs 44.7%, p=0.045). Adherent patients were also more likely to have frequent clinical visits yearly (median: 3.4 vs 2.5 per year, p=0.0033). They were more likely to be diagnosed with HCC during follow-up (26.2% vs 4.3%, p<0.0001).
Predictors of adherence to HCC surveillance
Table 2 presents predictors of imaging every 6 months. On multivariable analysis, age over the median age of 54 years (OR 1.74, 95% CI 1.38 to 2.18; p<0.0001), Asian ethnicity (OR 2.23, 95% CI 1.60 to 3.10; p<0.0001), decompensation (OR 2.40, 95% CI 1.85 to 3.11, p<0.0001) and having at least two clinical visits per year (OR 1.33, 95% CI 1.06 to 1.67, p=0.01) were predictors of adherence. Gender was not a significant predictor.
Predictors of adherence (every 6 months)
Characteristics of adherent versus non-adherent patients who developed HCC
A total of 228 (9.6%; adherent n=151 (66%); non-adherent n=77 (34%)) patients developed HCC during follow-up. As shown in table 3, adherent patients were less likely to have alpha-fetoprotein (AFP) levels >1000 ng/mL at the time of HCC diagnosis (5.8% vs 19.4%, p=0.003) and more likely to have smaller tumours (2.3 vs 3.3 cm, p=0.0020) and a Barcelona Clinic Liver Cancer (BCLC) stage of 0 or A (70.4% vs 38.7%, p=0.003). In addition, adherent patients were more likely to be within the Milan criteria for liver transplants (73.2% vs 54.8%, p=0.006) and the University of California, San Francisco (UCSF) criteria for liver transplants (87.9% vs 68.7%, p=0.001).27 28 They were also more likely to receive curative HCC treatment (43.6% vs 24.0%, p<0.005) (table 3).
Characteristics of patients with HCC overall and by adherent (every 6 months) versus non-adherent status
Survival analysis of patients who developed HCC
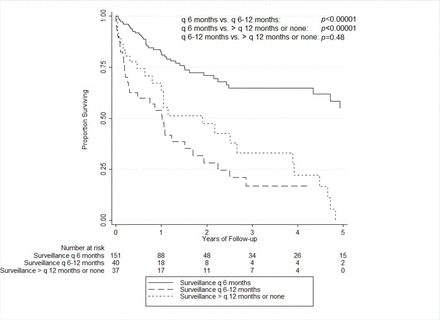
Of the 228 patients who developed HCC, the overall 5-year cumulative survival following HCC diagnosis was 33.6% (95% CI 23.6% to 43.9%). For patients adhering to surveillance every 6 months, the 5-year cumulative survival was 54.7% (95% CI 40.5% to 66.9%) compared with 6.5% (95% CI 1.3% to 18.0%) for non-adherent patients (p<0.00001). Comparing the adherent group (surveillance every 6 months) with the non-adherent subgroup with surveillance only every 6–12 months, there was a large and significant difference in their 5-year survival rates (54.7% (40.5%–66.9%)) vs 16.9% (6.1%–32.4%), p<0.00001). The 5-year survival rate for the non-adherent subgroup with surveillance more than every 12 months was 0% and there was no statistically significant difference in survival between the non-adherent subgroups (p=0.48) (figure 2).
Overall survival by adherence status.
By multivariable Cox proportional hazards model, every 6-month adherence (HR 0.34 95% CI 0.16 to 0.72, p=0.005) and curative HCC treatment (HR 0.32 95% CI 0.17 to 0.61 p=0.001) were associated with a 66% and 68% reduced risk of mortality, respectively (table 4). While every 6-month adherence was associated with a reduced risk of mortality, every 6–12 months adherence was not. In addition, age (HR 1.05, 95% CI 1.01 to 1.09, p=0.039) was associated with a 5% increase in mortality for every year of advancing age.
Cox proportional hazards model for mortality for patients with HCC
Discussion
In this large, retrospective cohort study of consecutive patients with CHC cirrhosis seen at a university centre, adherence to the 2000 EASL guidelines or the 2005/2011 AASLD HCC surveillance guidelines was poor, with only 44% receiving HCC surveillance at least every 12 months and only 24% receiving HCC surveillance every 6 months. This confirms a prior study that found that adherence to HCC surveillance among patients with chronic hepatitis B was poor and that even among those who were adherent at the beginning of follow-up, surveillance sharply decreased over the 5 years of follow-up, suggesting that adherence in chronically ill patients is difficult.29 Our findings are also consistent with prior studies on HCC surveillance of other high-risk populations, which found low rates of adherence.24 30
These findings are surprising since in 2011 AASLD updated their recommendations from surveillance every 6–12 months to every 6 months. Although the release of this update during this study may have contributed to the low rates of every 6-month surveillance, 56% of the patients in this study either had no imaging or underwent imaging less frequently than once a year, indicating poor adherence to either AASLD guideline.13 14 In addition, prior to the first AASLD guideline in 2005, the EASL 2000 guideline already recommended imaging every 6 months for patients with cirrhosis of any aetiology.12
Furthermore, the surveillance rate remained substandard over the 16 years of the study. Although older studies have found low rates of surveillance, many of these studies largely predated the 2011 AASLD guidelines that recommend every 6 month imaging. One might expect that the rate of every 6-month imaging in a US cohort would increase after the release of these guidelines. However, there was no significant increase in adherence over time, not even after 2011, despite both AASLD and EASL guidelines recommending every 6 -month surveillance during this time period. As such, suboptimal adherence to practice guidelines continues to be an important practice issue.
In addition, our study confirms that surveillance every 6 months does reduce mortality, as adherent patients experienced a 66% reduced risk of mortality. Furthermore, adherent patients were more likely to be diagnosed with significantly smaller tumours, making them significantly more likely to receive curative HCC treatment, leading to a 68% reduced risk of death. Our results confirm prior findings that earlier detection of HCC leads to an improved chance of receiving curative treatment and survival.25 31 32
A potential explanation of the low adherence rate to both EASL and AASLD guidelines is that though both guidelines have indicated specific patients who may benefit most from screening, these guidelines are based only on level three data as described by the National Cancer Institute. The level of evidence, then, may lead to confusion as to when to start surveillance.33 In fact, all patients in our study should have been considered to be at high-risk (CHC cirrhosis), yet only 24.4% received screening every 6 months.
Others have found that general practitioners’ lack of knowledge of the guidelines, risk factors for HCC and how to screen for HCC may also lead to decreased surveillance.21 22 34–36 On the other hand, compared with other providers, gastroenterologists and hepatologists have been found to be more likely to recommend guideline-concordant HCC surveillance practices, and the number of specialist (gastroenterology and infectious diseases) visits has also been associated with adherence.23 37
However, from our study, it appears that practitioners used other criteria to determine when to begin surveillance. In particular, we found that more frequent clinical visits, older age, Asian ethnicity and decompensated cirrhosis were significantly associated with imaging every 6 months. However, selection bias by providers or other sociomedical or cultural characteristics of the patient group may have impacted the decisions made, though more frequent clinical visits have been reported as increasing adherence in other studies.38 To overcome these potential confounders in our study, all patients were selected from a large university in a metropolitan area.
We also acknowledge that patient compliance with practitioners’ recommendations may have influenced the adherence rate. In fact, several recent studies revealed that patient-reported barriers include: time from clinic appointment and time of imaging study >180 days, longer distance from the hospital and fewer clinic visits. In one study, almost 50% of the patients believed that healthy diets exclude the need for HCC surveillance, and 34% believed that surveillance was unnecessary if they had normal physical examinations and/or lacked symptoms. Another potential barrier to adherence to HCC surveillance may be due to the asymptomatic nature of early stage disease, as shown by a study of patient self-reported data that found that lack of symptoms or discomfort was the second most common barrier to not receiving guideline-recommended HCC surveillance.38–40
Our study has some limitations. The first is that many published studies, to include this one, are not randomised controlled trials (RCTs), which could introduce bias. However, RCTs may not be appropriate at this time, especially in light of a recent study in which patients were polled about whether they would participate in a RCT for HCC surveillance—99.5% declined randomization and 88% elected non-randomised surveillance.41 Another limitation of the retrospective design is that adherence may be underestimated if imaging tests were performed at outside facilities without the results being sent to our study site. However, we also reviewed physician notes, which likely would mention if imaging had been done at outside facilities. Additionally, while our study included many Hispanic/Latino and Asian patients who are often under-represented, it included relatively few African-American patients.
Nevertheless, a strength of this study is that we used clinical data instead of survey data, which is prone to recall bias by physicians and patients, or electronic medical record (EMR) extraction, which can miss more imaging tests done at outside facilities and is more prone to classification bias in regards to cirrhosis and HCC. Furthermore, our study is a real-life cohort of consecutive patients with CHC, so selection bias and recall bias are significantly decreased.
Conclusion
Our study suggests that adherence to HCC surveillance guidelines has remained poor over time. In this large cohort study of patients with CHC cirrhosis (considered high-risk for HCC), adherence to the AASLD and EASL surveillance guidelines was seen in less than half of the patients. Adherence was poor over the course of the fifteen years of the study, with no significant improvement over time, not even after the 2011 AASLD guideline. Adherent patients were more likely to undergo curative treatment and experienced significantly better survival. Having at least two clinical visits a year was associated with a 33% higher chance of optimal HCC surveillance. However, more work is necessary to determine effective methods of improving knowledge of the guidelines and to overcome barriers to care.
References
Footnotes
Contributors SAT: planned and conducted the study, including collecting, analysing and interpreting the data; drafting the manuscript; approved the final draft submitted. AL, CZ and JH: data collection, interpretation and critical review of the paper; approved the final draft submitted. LY and SW: data collection and critical review of the paper; approved the final draft submitted. LH: data interpretation and critical review of the paper; approved the final draft submitted. MHN: developed study concept and design, planned and conducted the study, including collecting, analysing and interpreting the data; drafting the manuscript; approved the final draft submitted.
Funding The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests None declared.
Patient consent Detail has been removed from this case description/these case descriptions to ensure anonymity. The editors and reviewers have seen the detailed information available and are satisfied that the information backs up the case the authors are making.
Ethics approval Stanford University Institutional Review Board.
Provenance and peer review Not commissioned; externally peer reviewed.