Article Text
Abstract
Background There are no head-to-head randomised controlled trials (RCTs) comparing the effectiveness of biologics in ulcerative colitis (UC). We aimed to assess the cost-effectiveness of adalimumab, infliximab and vedolizumab as first-line agents to induce clinical remission and mucosal healing (MH) in UC.
Methods We constructed a decision tree based on a payer's perspective in the USA to estimate the first year costs of adalimumab, infliximab or vedolizumab to achieve clinical remission and MH in patients with moderate-to-severe UC. Transition probabilities were derived from ACT, ULTRA and GEMINI RCT data. Costs were derived from Medicare reimbursement rates and wholesale drug prices.
Results Assuming a biological-naïve cohort, infliximab 5 mg/kg every 8 weeks was more cost-effective ($99 171 per MH achieved) than adalimumab 40 mg every other week ($316 378 per MH achieved) and vedolizumab every 8 weeks ($301 969 per MH achieved) at 1 year. Non-drug administration cost of infliximab exceeding $1974 per infusion would make adalimumab more cost-effective. First-line UC therapy with vedolizumab would be cost-effective if the drug acquisition price was <$2537 for each 300 mg administration during the 1-year time horizon.
Conclusions If non-drug costs of infliximab administration are not excessive (<$2000), infliximab is the most cost-effective first-line biologic for moderate-to-severe UC. Exceeding this threshold infusion-related cost would make adalimumab the more cost-effective therapy. Considering its drug costs in the USA, vedolizumab appears to be appropriately used as a second-line biologic after antitumour necrosis factor failure.
- INFLAMMATORY BOWEL DISEASE
- ULCERATIVE COLITIS
- COST-EFFECTIVENESS
This is an Open Access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Statistics from Altmetric.com
Summary box
What is already known about this subject?
▸ Biological therapies, increasingly used in inflammatory bowel disease, are highly effective yet very costly.
▸ Adalimumab, infliximab and vedolizumab have been approved for the treatment of moderate-to-severe ulcerative colitis (UC), and they represent effective first-line therapy options for biological-naïve patients.
▸ The lack of head-to-head randomised controlled trials comparing biological therapies makes it difficult to assess the cost-effectiveness between treatment options.
What are the new findings?
▸ In most cases, infliximab is the most cost-effective first-line therapy option, but it is not cost-effective if per-infusion costs are >$1974.
▸ Since adalimumab does not incur non-drug infusion costs with its subcutaneous delivery, it is the most cost-effective first-line therapy option when infusion costs for competing biologics are high.
▸ Vedolizumab would achieve higher cost-effectiveness as first-line therapy if the drug cost were substantially lower. From a cost-effectiveness perspective, it is better positioned as a second-line biological therapy option in moderate-to-severe UC.
How might it impact on clinical practice in the foreseeable future?
▸ Understanding specific non-drug and drug cost variables that impact the cost-effectiveness of adalimumab, infliximab and vedolizumab could inform the selection of these therapies in clinical practice in a per-case basis and optimise the future value of using these therapies.
Introduction
Ulcerative colitis (UC) is a relapsing and remitting chronic disease characterised by bloody diarrhoea, abdominal pain and inflammatory ulcerations of the colonic mucosa that accounts for an estimated $2.7 billion in direct US healthcare spending annually with substantial indirect costs and work-related opportunity costs in affected patients.1–3 While biological agents, the newest and most effective treatments for moderate-to-severe UC, have dramatically improved patient outcomes, these costly therapies also account for a third of annual disease-attributable costs.4 The lack of head-to-head randomised controlled trials (RCTs) makes it difficult to compare the efficacy of these therapies. From a clinical perspective, ambiguity persists in selecting the most cost-effective first-line biological agent for moderate-to-severe UC.
Adalimumab, a self-injectable medication, and infliximab, an intravenous medication delivered via an outpatient infusion, are both antibodies to tumour necrosis factor-α (anti-TNFα) and the two most commonly used biological therapies for the treatment of moderate-to-severe UC. While adalimumab requires little to no drug administration costs, non-drug costs associated with infliximab infusions can be greater than the cost of the drug itself.5 It is unknown whether one of these anti-TNFα agents is more cost-effective than the other.
Recently, vedolizumab was added to the arsenal for potential first-line biologics for moderate-to-severe UC and received the Food and Drug Administration (FDA) approval in May 2014. Although adalimumab and infliximab are often considered as first-line agents in gastroenterology practices, it is unclear whether the promising initial results of vedolizumab as an intestinal-specific α4β7 integrin inhibitor may be a more cost-effective first-line option.
In this study, we hypothesised that adalimumab, with its subcutaneous and self-injectable delivery, is most cost-effective in inducing and maintaining remission in moderate-to-severe UC. The objective of our study was to compare the cost required for clinical remission and mucosal healing (MH)6–8 when using adalimumab, infliximab or vedolizumab as a first-line biological therapy for moderate-to-severe UC. The secondary aim of this analysis was to assess the threshold drug and administration costs for each of these therapies when considering cost-effectiveness as a first-line biological agent.
Methods
Clinical remission and mucosal healing as a primary outcome measure
We built a decision analytic tree in TreeAge Pro 2015 (Williamstown, Massachusetts, USA) to compare the cost-effectiveness of first-line treatment with adalimumab, infliximab or vedolizumab for inducing MH at 1 year in biological-naïve patients with moderate-to-severe UC. The model was based on a third party's perspective in the USA. The primary outcome was clinical remission and MH achieved after 1 year of treatment. In today's era of biologics, achieving MH in the first year of biological treatment has become a top clinical priority in inflammatory bowel disease (IBD) practice, and it is now a standard outcome measure of interest for phase 3 FDA drug trials. In keeping with the data from published randomised clinical trials (RCTs) for IBD biological therapies, we defined MH as a Mayo Clinic endoscopic subscore of 0 or 1.
Derivation of transition probabilities and costs
Transition probabilities were derived from each drug's published RCTs and adjusted for comparison based on the assumption of a universally equivalent placebo group and a composite placebo treatment effect. Costs estimates were derived from the 2014 Medicare Physician Reimbursement Rates (HHS) and Red Book wholesale prices (AWP), with drug costs calculated for an adult patient with UC at the base case weight of 70 kg. Table 1 outlines the probabilities and costs used in the model.
Model parameters
Competing strategies
Competing strategies in our model, as depicted in figure 1, were based on ACT, ULTRA and GEMINI trials for infliximab, adalimumab and vedolizumab, respectively. The four strategies evaluated in this model were infliximab 5 mg/kg at weeks 0, 2 and 6, and then every 8 weeks for maintenance; infliximab 10 mg/kg at weeks 0, 2 and 6, and then every 8 weeks; adalimumab 160 mg at week 0, 80 mg at week 2 and 40 mg at week 4, and then 40 mg every other week (EOW) beginning at week 4 for maintenance; vedolizumab 300 mg at weeks 0, 2 and 6, and then every 8 weeks for maintenance.
Model schematic of competing strategies. *Notes: number of weeks to 1 year. ADA, adalimumab; EOW, every other week; IFX, infliximab; VDZ, vedolizumab.
ACT, ULTRA and GEMINI trials
Since our base case assumes a biological-naïve group, adjustments were made for the percentage of biological-naïve patients actually participating in each trial arm. The ACT infliximab trials enrolled only biological-naïve patients. Although the ULTRA adalimumab and GEMINI vedolizumab trials reported the percentages of patients who were biologic-naïve, only the ULTRA adalimumab trial published stratified data allowing an estimation of the effect of prior anti-TNFα drug exposure. Since the effect of previous biological failure on predicted drug efficacy is significant (biological-naïve patients responded at an average of 40% better across all markers and time points), we considered it important to account for this difference in our model.
We made the assumption that vedolizumab treatment would be less effective in patients who were previously exposed to anti-TNFα agents. Since drug effectiveness was not stratified based on biological exposure or non-exposure in the GEMINI vedolizumab trials, we assumed that vedolizumab treatment, owing to its different, non-anti-TNFα mechanism of action, would not experience more than a 40% decrease in effectiveness in patients with previous anti-TNFα treatment (in clinical practice today, the rationale for switching to vedolizumab from anti-TNFα agents has been the gut-specific blockade of intestinal lymphocyte trafficking—a completely different mechanism of action than the anti-TNFα monoclonal antibodies). To account for the drugs' mechanistic differences, we tested this described variable in our sensitivity analyses. Regardless of a 10–40% diminution of effectiveness of vedolizumab among anti-TNFα exposed patients, overall results of cost-effectiveness did not vary significantly in the model. In order to approximate an all-naïve population undergoing treatment, the final model assumed that vedolizumab in biological-naïve patients may have up to 20% increased effectiveness in comparison to published data, since approximately half of the participants in the vedolizumab trial had prior anti-TNFα exposure.
Extrapolation of unreported GEMINI trial data
The GEMINI vedolizumab trial did not publish results for patients without clinical response after 6 weeks of treatment. Since our clinical experience and emerging data have found vedolizumab slower to act compared with traditional anti-TNFα agents, we did not assume that these unreported patients had primary treatment failure at 6 weeks. Testing a wide range of possible clinical responses from as low as 10% to as high as 90% did not vary the results of the model significantly.
Sensitivity analyses
We performed both deterministic and probabilistic sensitivity analyses on all probabilities and costs in the model (table 1). One-way deterministic sensitivity analyses were performed to identify threshold point estimates for individual probabilities and costs—the precise cut-off value when a previously cost-effective strategy becomes cost-ineffective (or vice versa). Importantly, drug costs of the three biologics were tested separately to determine the cut-off acquisition price needed to achieve cost-effectiveness over competing biological strategies. Probabilistic sensitivity analysis was performed to test the durability of our results with variability in the parameters.
Results
Cost-effectiveness of adalimumab versus infliximab versus vedolizumab
Table 2 shows the cost-effectiveness of the strategies after 1 year of treatment in a 70 kg biological-naïve adult patient with moderate-to-severe UC in the USA. Our model showed that infliximab 5 mg/kg every 8 weeks was more cost-effective ($99 171 per MH achieved) than adalimumab 40 mg EOW ($316 378 per MH achieved) and vedolizumab every 8 weeks ($301 969 per MH achieved). Infliximab 10 mg/kg every 8 weeks achieved a cost-effectiveness of $123 653 per MH achieved, but an additional $1.24 million is required to achieve one additional patient achieving MH after 1 year of treatment. This indicates that for the majority of scenarios at the cohort level, only a small incremental benefit in direct costs would be seen if 10 mg/kg every 8 weeks were the standard of care.
Summary of cost-effectiveness after 1 year of treatment
Threshold analyses
Administration costs of infliximab
We originally hypothesised that adalimumab would be cost-effective given its lower non-drug costs; however, infliximab was more cost-effective due to its superior effectiveness and lower drug costs. However, it is important to note that we used Medicare reimbursement data for non-drug costs associated with infliximab administration costs ($115.84 per infusion). Privatised reimbursement costs for infliximab infusions at outpatient infusion centres in the USA are likely to far exceed Medicare's administration reimbursement for a 3 h infusion, and there are no published data describing the variability of non-drug infusion-related costs across different health systems in the USA. Clinical observation would speculate that outpatient infusion units tend to be less expensive than hospital-based infusions. We found that infliximab administration costs of more than $1974 would make adalimumab a more cost-effective option (table 3).
Threshold analysis
Drug costs of adalimumab and vedolizumab
Table 3 summarises the key threshold costs impacting cost-effectiveness between biologics. In our analysis, drug cost was the primary driving factor in cost-effectiveness for adalimumab and vedolizumab. Our threshold analysis showed that adalimumab would have to be <$1156, and vedolizumab would have to be <$2537 to be considered the cost-effective first-line biologic over the 1-year time horizon.
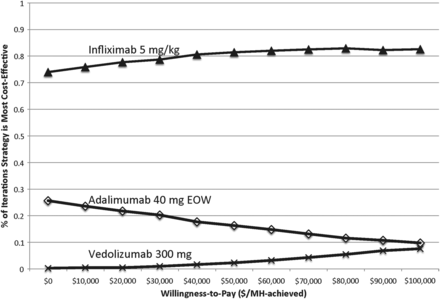
Probabilistic sensitivity analysis
Figure 2 shows the cost-effectiveness acceptability curve after 1000 independent simulations, indicating the probability of each strategy achieving optimal cost-effectiveness at various willingness-to-pay thresholds per MH achieved. Of note, infliximab administration cost was not varied in this probabilistic sensitivity analysis since the previous threshold analysis showed a clear inflection price at ∼$2000 (for non-drug costs) at which point choosing adalimumab would be more cost-effective than infliximab.
Cost-effectiveness acceptability curve indicating the proportion of simulations at which each strategy is optimal at various willingness-to-pay thresholds. EOW, every other week; MH,mucosal healing.
Discussion
We performed a cost-effectiveness analysis to determine if adalimumab, infliximab or vedolizumab would be most cost-effective as a first-line biological agent in moderate-to-severe UC. Although we identified infliximab 5 mg/kg every 8 weeks as the most cost-effective first-line agent, variations in key cost parameters for each of the three biologics would impact overall cost-effectiveness.
For infliximab, the most important consideration is the non-drug cost associated with its administration. We found that at infusion-related costs over $2000—$1974 to be exact—infliximab would not be the most cost-effective first-line biologic. Since adalimumab is not subject to non-drug costs with its subcutaneous delivery, it becomes the more cost-effective therapy option if the non-drug infliximab costs are high. In our model, a Medicare reference of $116 for reimbursement of infliximab administration was used. Since private insurance reimbursements are not readily available for comparison across many health systems, price transparency around non-drug costs becomes a key topic of discussion when attempting to compare the cost-effectiveness between potential first-line biological therapies in UC. In the US private sector, it is probable that infliximab administration costs are highly variable, most likely exceeding the $2000 non-drug cost per infusion cost-effectiveness threshold.9 Of note, vedolizumab also requires infusion-related costs and would be subject to similar non-drug cost limitations.
In our analysis, since the effectiveness of vedolizumab after 1 year for MH was not superior to infliximab, vedolizumab's drug cost became an important cost parameter for its cost-effectiveness. We found that vedolizumab 300 mg every 8 weeks would achieve superior cost-effectiveness if the 300 mg dose is priced <$2537, compared with its current wholesale cost of $5788. In real life, vedolizumab is currently more likely to be used as a second-line biologic, given its alternative mechanism of action, after anti-TNFα failure or loss or response after infliximab or adalimumab use. In such clinical scenarios, there would be less application of a fixed threshold drug cost since the ‘first-line’ therapy indication would not apply to vedolizumab. For adalimumab 40 mg EOW, a premade syringe price <$1156 would be cost-effective, compared with its current wholesale cost of $1748.
Although there are notable retrospective reports evaluating the real-world effectiveness between biologics, the absence of comparative effectiveness trials in biologics contributes to the clinical ambiguity of selecting the most cost-effective first-line biological therapy in IBD.10 ,11 However, despite the utility of our extrapolated, head-to-head comparison of effectiveness and direct costs, the importance of a patient-centred, individualised therapy plan cannot be overemphasised. Although we were able to highlight key cost parameters driving cost-effectiveness of each of the three biologics, gastroenterologists’ considerations for patient preferences, differences in access to care, adherence levels to agreed-on therapy plans and other individual-level considerations all contribute to providing high-value care for patients with IBD. The impact of such patient-oriented care strategies are less apparent in calculating cost-effectiveness, but no less important.
Related to the topic of considering individual patient needs, our model is limited to published data in ACT, ULTRA and GEMINI trials. Real-life dosing for biologics (especially infliximab) is often personalised, falling outside the typical strategies described in RCTs. Our model considered standard maintenance dosing for infliximab and adalimumab, and it is not able to precisely quantify the incremental benefit and cost of dose escalations and increased drug frequency, although inferences can be made about accruing costs and health benefits based on published studies.12–14 Of note, the cost-effectiveness of a dose escalation from 5 to 10 mg/kg every 8 weeks is appreciated in the model since the latter dosing was part of the ACT infliximab trial. The fact that 10 mg/kg every 8 weeks is not cost-effective highlights the small cohort-level benefit and substantial additional cost if 10 mg/kg every 8 weeks were chosen over the 5 mg/kg every 8 weeks at initiation of therapy and continued over the 1-year time horizon. This does not imply that using higher dosing or increased frequency (eg, every 4–6 weeks) is not cost-effective in developing clinical scenarios (eg, loss of response, drug autoantibody formation). It is also important to note that US drug costs are not comparable to other global markets such as Europe or Asia, especially since early data show that competing biosimilars are pushing down the cost of biologics.15
A final limitation to discuss in our analysis is the underlying assumptions needed to generate unpublished data on vedolizumab. As noted in the methods, we considered a wide range of probabilities (ie, as low as 10% and as high as 90%) that estimate the actual clinical response rate to vedolizumab after initial non-response after 6 weeks of therapy. Also, since nearly half of the GEMINI vedolizumab trial enrolled anti-TNFα exposed patients, we assumed a 10–40% (median of 20%) diminution of effectiveness of vedolizumab among these patients. While we acknowledge that these are important unknown variables, the overall results of the model did not change despite testing the range of potential probabilities. As stated above, the main driving variable of vedolizumab's cost-effectiveness as first-line therapy was its cost.
In summary, while we show that infliximab is the most cost-effective first-line biological agent for patients with moderate-to-severe UC, high non-drug costs associated with infusions in the USA may prevent cost-effective clinical use in real-world practice with adalimumab representing a viable alternative option. Vedolizumab appears appropriate as a second-line anti-TNFα rescue agent and is priced accordingly in the USA to uniquely fill this clinical need.
Acknowledgments
The authors acknowledge the support of the Stanford Medical Scholars Fellowship Program (to LY) and the National Institutes of Health (K08 DK094868A to KTP).
References
Footnotes
Contributors LY collected and analysed the data and drafted the initial manuscript. BL contributed to the design of the study and critically revised the paper. KTP, the guarantor of the article, conceptualised and designed the study, analysed the data, and drafted and revised the manuscript. All authors approved the final version of the article, including the authorship list.
Funding National Institute of Diabetes and Digestive and Kidney Diseases (grant no. DK094868A).
Disclaimer The manuscript contents are solely the responsibility of the authors and do not necessarily represent the official views of Stanford University School of Medicine or the National Institutes of Health.
Competing interests None declared.
Provenance and peer review Not commissioned; externally peer reviewed.
Data sharing statement No additional data are available.