Article Text
Abstract
Objective Circulating microRNAs (miRNAs) are promising biomarkers for the early detection of cancers. This study aimed to address potential circulating miRNAs to monitor the progression from Barrett’s oesophagus (BO) to oesophageal adenocarcinoma (OAC).
Design We comprehensively analysed tissue and serum miRNA expression profiles of BO mice model (L2-interleukin-1β (IL-1β) mice) using microarray analysis. To validate the data from mice, a published dataset of human plasma miRNAs, consisting of eight patients with OAC, eight with BO and six healthy controls, was used (GSE51410).
Results We identified 20 upregulated miRNAs and 44 downregulated miRNAs both in tissues and in sera of 46-week-old mice compared with 28-week-old mice. Two of the 20 miRNAs (miR-128-3 p and miR-328-3 p) were upregulated, and five of the 44 miRNAs (miR-143-3 p, miR-144-3 p, miR-15a-5p, miR-1-3 p and miR-133b) were downregulated in plasma of patients with OAC compared with plasma of patients with BO. Receiver operating characteristic curve analysis revealed that a prediction index calculated by the above-mentioned seven miRNAs could discriminate between patients with OAC and those without OAC with the area under the curve of 0.91, sensitivity of 1 and specificity of 0.75.
Conclusions Levels of the seven circulating miRNAs may represent the tissue miRNA levels and could be promising non-invasive biomarkers to evaluate the carcinogenic process of BO.
- barrett's oesophagus
- oesophageal cancer
- rna expression
This is an Open Access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Statistics from Altmetric.com
Summary box
What is already known about this subject?
Risk stratification and early detection of oesophageal adenocarcinoma must improve the prognosis.
Circulating microRNAs (miRNAs) are potential biomarkers for the early detection of cancers.
What are the new findings?
Seven circulating miRNAs (miR-128-3 p, miR-328-3 p, miR-143-3 p, miR-144-3 p, miR-15a-5p, miR-1-3 p and miR-133b) were identified as promising biomarkers to evaluate the carcinogenesis from Barrett’s oesophagus to oesophageal adenocarcinoma using mouse and human circulating miRNA datasets.
How might it impact on clinical practice in the foreseeable future?
Our results would assist the development of blood-based future diagnostic strategy for the detection of early-stage oesophageal adenocarcinoma.
Introduction
Oesophageal adenocarcinoma (OAC) is one of the most dramatically increasing malignant diseases in Western countries. The incidence of OAC has increased around six times in the last four decades.1 Well-known causal factors of OAC are the presence of gastro-oesophageal reflux diseases and obesity and the absence of Helicobacter pylori infection.2–5 Barrett’s oesophagus (BO), which is histologically characterised by the replacement of the normal stratified squamous epithelium of the oesophagus with a columnar epithelium, is considered to be a precursor lesion of OAC.3 4 To improve patient survival and reduce disease burden, early-stage detection or preventing the progression of OAC from BO constitute the best options. Although endoscopic surveillance for patients with BO has been usually conducted, an absolute annual risk for the development of OAC from BO is only 0.12%.6 Therefore, considering the invasiveness, recommending conventional endoscopic screening procedure has been deemed controversial.7 Thus, identification of non-invasive risk stratification biomarkers to determine the risk of progression from BO to OAC may improve disease outcome and render patient management more efficient.
MicroRNAs (miRNAs) are a class of small non-coding endogenous RNAs, which are 18–25 nucleotides in length. They regulate gene expression at the post-translational level by guiding the RNA-induced silencing complex to miRNA target sites in the 3′-untranslated region of mRNA, which leads to mRNA degradation or the inhibition of translation.8 miRNAs participate in many essential biological processes, including proliferation, differentiation, apoptosis, necrosis, autophagy and stress responses. miRNAs also play a crucial role in cancer pathogenesis through their functions as oncogenes or tumour suppressors depending on their gene targets.9–11 In fact, aberrant expression of various miRNAs has been reported during the transformation from BO to OAC.12–20
More importantly, recent evidence has emerged that miRNAs can be secreted from cells, are in circulation or can be taken up by other cells.21 22 Because unprotected miRNAs are sensitive to degradation by RNases present in blood, stably existing circulating miRNAs are assumed to be bound to either RNA-binding proteins23 or high-density lipoproteins24 or are encapsulated within extracellular vesicles.22 Circulating miRNAs are desirable candidates as non-invasive biomarkers for diagnosis, prognosis and prediction in cancer management.25 26 Therefore, circulating miRNA profiling could also improve the risk stratification for the progression of BO to OAC. However, because expression levels of circulating miRNAs are affected by various systemic conditions such as age, sex and obesity,27 28 it is difficult to clarify just by human observational study whether OAC-specific circulating miRNA expression profiles are derived from the tissue expression profiles of OAC. Therefore, we investigated circulating miRNA levels in a mouse model of BO. Subsequently, we validated the presence of circulating miRNA using a published human circulating miRNA dataset.
Methods
Animals
L2-IL-1β mice were kindly provided by Dr Timothy C Wang of Columbia University Medical Center, New York. L2-IL-1β mice that are 28 and 46 weeks old (n=2 for each) were sacrificed by cervical dislocation, their oesophagus and stomachs were dissected and sera were obtained. Animal experiments were performed under the approval of the Animal Research Committee of Keio University School of Medicine.
Histological examination
Specimens of the oesophago-gastric junction in mice were fixed in 10% neutralised buffered formalin, embedded in paraffin and sectioned with a thickness of 5 µm. Sections were depleted of paraffin, and then rehydrated in a graded series of ethanol solutions. Sections were stained with H&E and observed with a BZ-X700 microscope (Keyence, Tokyo, Japan).
Total RNA extraction
Total RNA, including small RNA, was extracted using the mirVana miRNA isolation kit (Ambion, Austin, Texas, USA) for tissue samples, and miRNeasy Serum/Plasma Kit (Qiagen, Hilden, Germany) was used for serum samples in accordance with the manufacturer’s instructions. The quality of total RNA was checked on a NanoDrop 1000 spectrophotometer (Thermo Scientific, Wilmington, Delaware, USA) and a 2100 Bioanalyzer (Agilent Technologies, Santa Clara, California, USA). The amount of total RNA of tissue samples was evaluated using NanoDrop 1000, whereas that of serum samples was evaluated using 2100 Bioanalyzer because the RNA concentration was low.
miRNA expression analysis
Comprehensive miRNA expression analysis was performed with 250 ng of total RNA from tissue samples and 40 ng of total RNA from serum samples using a 3D-Gene miRNA labelling kit and a 3D-Gene Mouse miRNA Oligo Chip (Toray Industries, Tokyo, Japan), which are designed to detect 1265 miRNA sequences registered in miRBase release 19 (http://www.mirbase.org/). A miRNA was considered to be present if the corresponding microarray signal was higher than (mean +2 × SD] the signal of the negative controls, of which the 5% of the top and bottom ranked were eliminated by signal intensity. Once a miRNA was considered present, the mean signal of the negative controls, of which the 5% of the top and bottom ranked were eliminated by signal intensity, was subtracted from the miRNA signal. To normalise the signals among microarrays tested, a global median normalisation method was applied.
Statistical analysis
Hierarchical clustering analysis of miRNAs were performed using Partek Genomics Suite 6.6 (Partek, St. Louis, Missouri, USA). A prediction model using multiple miRNAs associated with the presence of OAC was constructed by logistic regression analysis. The area under the receiver operating characteristic curve (AUC), diagnostic sensitivity and specificity were calculated by receiver operating characteristic (ROC) curve analysis. Two-sided p values of <0.05 were considered statistically significant. Statistical analyses were performed using IBM SPSS Statistics V.22.
Results
Detecting circulating miRNAs that represent miRNA expression profiles of the progression of BO to OAC in mice
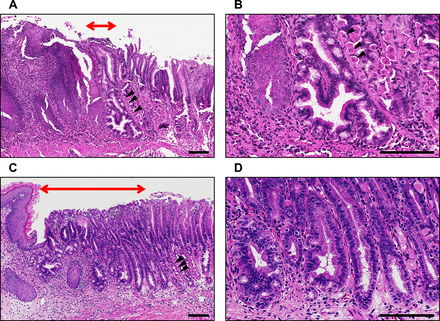
We used human interleukin-1β (IL-1β) transgenic mice (L2-IL-1β mice),29 generated by targeting the expression of human IL-1β to the oesophagus using the Epstein-Barr virus promoter as a model of BO. Histopathology and gene signatures of this model are reported to resemble human BO. We confirmed that metaplastic glands could be observed at the squamocolumnar junction in 28-week-old L2-IL-1β mice (figure 1A,B). In 46-week-old L2-IL-1β mice, multiple dysplastic glands could be observed at the squamocolumnar junction, although OAC was not developed (figure 1C,D).
Representative histopathology at the squamocolumnar junction in L2-IL-1β mice; (A) histopathology in 28-week-old mice; (B) magnified image of A; (C) histopathology in 46-week-old mice; (D) magnified image of C. Red double-headed arrows, area of metaplastic or dysplastic glands; black arrow heads, gastric parietal cells; scale bars=100 µm. IL-1β, interleukin-1β.
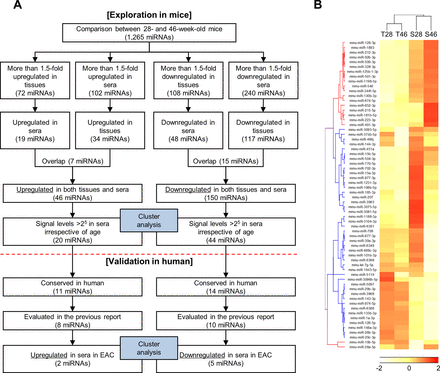
We comprehensively analysed tissue and serum miRNA expression profiles of 28-week-old and 46-week-old L2-IL-1β mice using microarray analysis (online supplementary figure S1). Results of miRNA microarrays have been deposited in the National Center for Biotechnology Information Gene Expression Omnibus database with accession code GSE100390. By comparing between 28-week-old and 46-week-old mice, potentially deregulated miRNAs during the progression of BO were investigated (figure 2A). First, we selected all the deregulated miRNAs with fold change of >1.5 between the values of 28-week-old and 46-week-old mice either in tissues or in sera. Second, miRNAs with inversely altered levels in tissues and sera due to ageing were excluded. Finally, miRNAs with levels of <25 in sera after normalisation were excluded as it limits the data reliability of lower signal levels in serum because the level of total RNA is relatively low in the serum. Using this strategy, we identified 20 upregulated miRNAs and 44 downregulated miRNAs in both tissues and sera (figure 2B).
Supplementary file 1
(A) Flow chart illustration of the experimental design; (B) hierarchical clustering analysis based on selected tissue and serum miRNA profiles obtained from 28-week-old and 46-week-old L2-IL-1β mice. Twenty upregulated and 44 downregulated miRNAs in both tissues and sera in 46-week-old mice compared with 28-week-old mice were selected. miRNAs, microRNAs; T28, tissue miRNAs obtained from 28-week-old mice; T46, tissue miRNAs obtained from 46-week-old mice; S28, serum miRNAs obtained from 28-week-old mice; S46, serum miRNAs obtained from 46-week-old mice.
Validation of circulating miRNAs using published human dataset
To expand the mouse result to human, we excluded 39 miRNAs that are not evolutionally conserved in humans (figure 2A). Subsequently, we validated the data from mice using a published dataset of human plasma miRNAs, consisting of eight patients with OAC, eight with BO and six healthy controls (GSE51410).30 Our primitive aim was to identify biomarkers for human early stage OAC, namely intramucosal carcinoma and high-grade dysplasia. However, circulating miRNA profiles in patients with high-grade dysplasia have not been publicly available yet. Therefore, we used dataset of OAC instead of high-grade dysplasia. Since it is necessary that the biomarker of high-grade dysplasia can also detect OAC, validation using dataset of OAC is thought to be meaningful for the identification of biomarkers of high-grade dysplasia.
This dataset analysed plasma expression levels of 175 miRNAs that are present in human plasma samples using Serum/Plasma Focus miRNA PCR panels (Exiqon, Vedbaek, Denmark). Among 25 miRNAs that were identified in mice and conserved in humans, seven miRNAs could not be evaluated because they were not included in the PCR panels. Therefore, plasma levels of 18 miRNA were compared between patients with OAC and those with BO. Of the eight miRNAs that were upregulated in 46-week-old mice compared with 28-week-old mice, two miRNAs were also upregulated in patients with OAC compared with those with BO. Of the 10 miRNAs that were downregulated in 46-week-old mice compared with 28-week-old mice, five miRNAs were also downregulated in patients with OAC compared with those with BO. Thus, seven circulating miRNAs were considered as potential candidates of non-invasive biomarkers for the progression of BO to OAC (table 1).
Circulating microRNA candidates that represent the development of OAC from BO
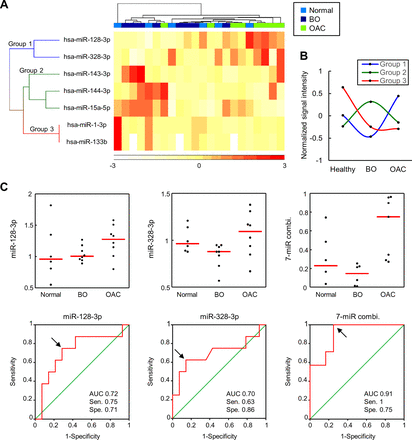
The clustering heat map revealed more explicit segregation of OAC samples from healthy or BO samples (figure 3A). In addition, seven miRNAs could be categorised into three groups, namely, miR-128-3 p and miR-328-3 p (group 1); miR-143-3 p, miR-144-3 p and miR-15a-5p (group 2); and miR-1-3 p and miR-133b (group 3), according to their expression profiles (figure 3A). Levels of miRNAs in group 1 were upregulated, whereas levels of miRNAs in groups 2 and 3 were downregulated during the progression from BO to OAC (figure 3B). Levels of miRNAs in group 2 were the highest in patients with BO, whereas levels of miRNAs in group 3 were the highest in healthy controls (figure 3B). We determined the accuracy of these seven miRNAs in discriminating patients with OAC from those without OAC (healthy and BO) using the ROC curve analysis (table 1). Levels of miRNAs in group 1 could achieve the AUC of more than 0.7 to discriminate patients with OAC from those without OAC, although the discriminating accuracy was not statistically significant. When we calculated a predictive index by the combination of seven miRNAs, this index could discriminate patients with OAC significantly. The respective data and ROC curves of miRNAs in group 1 and a predictive index are shown in figure 3C. By using the prediction index, patients with OAC could be discriminated accurately with the AUC of 0.91, sensitivity of 1 and specificity of 0.75.
(A) Hierarchical clustering analysis based on human plasma miRNA profiles obtained from patients with OAC (n=8), with BO (n=8) and without BO (n=6). (B) The average of normalised signal intensity used in the heat map. (C) Dot plots show that plasma levels of miR-128-3 p and miR-328 and a predictive index calculated from the levels of seven miRNAs (miR-128-3 p, miR-328-3 p, miR-143-3 p, miR-144-3 p, miR-15a-5p, miR-1-3 p and miR-133b) were higher in patients with OAC (upper panels). ROC curves show the discrimination accuracy for discriminating patients with OAC from those without OAC using plasma levels of miR-128-3 p, miR-328 or a predictive index calculated from levels of seven miRNAs (lower panels). Red bar, median; black arrows, optimum cut-off points to calculate sensitivity and specificity. AUC, area under the receiver operating characteristic curve; BO, Barrett’s oesophagus; OAC, oesophageal adenocarcinoma; Sen., sensitivity; Spe., specificity.
Discussion
We identified seven miRNAs in mice tissue and serum samples with expression levels that were compatible with the levels in human plasma. Hierarchical clustering analysis revealed that these seven miRNAs could be categorised into three clusters: miR-128-3 p and miR-328-3 p (group 1); miR-143-3 p, miR-144-3 p and miR-15a-5p (group 2); and miR-1-3 p and miR-133b (group 3). Levels of miRNAs in group 1 were upregulated in OAC compared with healthy controls or patients with BO and could discriminate OAC from healthy controls or BO comparatively better than levels of miRNAs in groups 2 and 3. In addition, by combining groups 1, 2 and 3, the discriminating accuracy could be enhanced, indicating that miRNAs in groups 2 and 3 need to be investigated further as potential candidates of biomarkers.
Although the role of miRNAs has not been investigated in the present study, we could hypothesise its role based on previous studies. In line with our results, miR-128-3 p is upregulated in colorectal, pancreatic and gastric adenocarcinoma tissues.31 32 However, in prostate cancer, glioma and non-small cell lung cancer (NSCLC), miR-128-3 p is downregulated.33–35 Tumour-suppressive properties have been explained by the inhibition of Bmi-1.35 The transcription factor Bmi-1 is essential for promoting self-renewal of several types of normal and cancer stem cells.36 Because Bmi-1 is overexpressed in gastric and pancreatic cancer,37 miR-128-3 p may be compensatory upregulated to suppress Bmi-1 expression in adenocarcinoma tissues.
Tissue expression levels of miR-328-3 p have been reported to be higher in NSCLC with brain metastasis than in NSCLC without brain metastasis.38 Functional analysis showed that miR-328-3 p has a role in conferring migratory potential to NSCLC cells, working in part, through the upregulation of the protein kinase C alpha gene. Circulating miR-328 has also been reported as a biomarker to discriminate early stage NSCLC from healthy controls with an AUC of 0.82.39 However, levels of miR-328-3 p are downregulated in colorectal cancer, and low miR-328-3 p expression tends to correlate with increased number of cancer stem-like cells.40 CD44, a well-known cancer stem cell marker, is a direct target of miR-328-3 p.41–44 Thus, both miRNAs in group 1 play a tumour-suppressive role through the inhibition of stem cell-like properties of the cancer cells. Upregulation of these miRNAs may be a compensatory reaction during the carcinogenesis of BO.
miR-143-3 p, miR-144-3 p and miR-15a-5p in group 2 are assumed to be tumour suppressors. Tissue levels of miR-143-3 p have been reported to be downregulated in colorectal cancer.45 Overexpression on miR-143-3 p suppresses tumour growth through the direct inhibition of the KRAS and ERK5 genes.46 47 Downregulation of miR-144-3 p is associated with colorectal cancer progression via activation of the mechanistic target of rapamycin signalling pathway.48 miR-15a-5p is a member of the miR-15 family, which includes miR-16-1, miR-16-2, miR-195 and miR-497. This cluster is known to suppress cell cycle through the inhibition of several oncogenes such as E2F1 and BCL2.49–51 Of note, circulating levels of group 2 miRNAs were elevated during the development of BO compared with those in healthy controls (figure 3B). Cabibi et al 52 also reported that tissue and circulating miR-143-3 p levels are elevated during metaplastic transformation of the oesophagus, suggesting that group 2 miRNA may be associated with intestinal differentiation.
miR-1-3 p and miR-133b, categorised in group 3, are known as muscle-specific miRNA and exhibit tumour-suppressive functions.53 54 In L2-IL-1β mice, human IL-1β is overexpressed only in the squamous cell layer of the oesophagus. Downregulation of oesophageal tissue levels of miR-1-3 p and miR-133b in this mouse model indicated that IL-1β in mucosal layer may affect the muscle layer, leading to the deregulation of muscle-specific miRNAs. We have previously reported that miR-1-3 p, miR-133a and miR-133b levels are decreased by H. pylori infection in mice gastric tissues, leading to impaired gastric motility.55 Because IL-1 expression is enhanced in gastric mucosa by H. pylori infection,56 57 the deregulation of muscle-specific miRNAs in these two animal models may be due to the same aetiology.
So far, several other circulating miRNAs have been employed to detect OAC (table 2).30 52 58–60 In addition, some miRNAs have been reported as prognostic biomarkers of OAC. Zhai et al 59 reported that a combination of two circulating miRNA (low miR-3935 expression and high miR-4286 expression) risk score exhibits greater risk for worse overall survival (HR=2.22) than the independent levels of high miR-3935 expression or low miR-4286 expression. Odenthal et al 61 reported that low miR-302c and high miR-222 expression is significantly correlated to worse overall survival (log-rank test, p<0.01). Moreover, we have previously reported that miR-222 expression levels are enhanced during exposure to bile acid in the oesophageal epithelial cells, which leads to the proteasomal degradation of tumour-suppressive protein Cdx2.19 62 Therefore, it would be reasonable to conclude that high levels of miR-222 are associated with poor prognosis. However, these reports are small-scale pilot studies, including our study; therefore, well-validated large-scale biomarker studies are warranted.
Previously reported circulating microRNA (miRNA) for the diagnosis of OAC and/or BO
Since circulating miRNA status in patients with high-grade dysplasia has not been reported, it has been unknown that our results also fit for the biomarker of high-grade dysplasia. According to reported tissue miRNA profiles, miRNA signatures of high-grade dysplasia were similar to those of OAC as compared with the signatures of non-dysplastic BO and normal squamous epithelium.63 Interestingly, tissue miRNA profiles of BO adjacent to high-grade dysplasia were also similar to those of high-grade dysplasia and OAC. Therefore, we could expect that circulating miRNA profiles of high-grade dysplasia might also be similar to those of OAC. To consider early-stage biomarkers for OAC, this issue must be intensively evaluated in future.
In conclusion, seven circulating miRNAs (miR-128-3 p, miR-328-3 p, miR-143-3 p, miR-144-3 p, miR-15a-5p, miR-1-3 p and miR-133b), consisting of three different characteristic miRNA groups, were identified as promising non-invasive biomarkers to evaluate the carcinogenesis from BO to OAC. However, our data were not based on enough number of samples. Although confirmation of data reproducibility and further prospective validations are needed, accumulating knowledge about circulating miRNAs will change future medical practices.
Acknowledgments
We would like to thank Dr Timothy C Wang for providing L2-IL-1β mice and Hitoshi Tsugawa, Hideki Mori, Tsuyoshi Yamane and Yuki Kashiwazaki for technical supports and valuable advices.
References
Footnotes
Contributors Both authors conceived and designed the experiments. JM performed the experiments and analysed the data. JM wrote the paper, and HS revised the paper.
Funding This study was supported by a Grant-in-Aid for Young Scientists (B) (26860527; to JM), a Grant-in-Aid for Scientific Research B (16H05291; to HS) from the Japan Society for the Promotion of Science (JSPS) (https://www.jsps.go.jp/english/), MEXT-Supported Program for the Strategic Research Foundation at Private Universities (S1411003; to HS) (http://www.mext.go.jp/english/pressrelease/1317526.htm), a grant from Takeda Science Foundation (to JM), the Princess Takamatsu Cancer Research grants (to HS) and the Medical School Faculty and Alumni Grant from Keio University Medical Science Fund (to JM and HS).
Disclaimer The funders have no roles in study design, data collection, data analysis, data interpretation or drafting the manuscript. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Competing interests During the last 2 years, HS received scholarship funds for the research from Daiichi-Sankyo Co., EA Pharma Co., Otsuka Pharmaceutical Co. Ltd and Tsumura Co. and received service honoraria from Astellas Pharma Inc., Astra-Zeneca K.K., EA Pharma Co., Otsuka Pharmaceutical Co. Ltd, Daiichi-Sankyo Co., Takeda Pharmaceutical Co. Ltd, Mylan EPD, Co., Tsumura Co. and Zeria Pharmaceutical Co. Ltd.
Provenance and peer review Commissioned; externally peer reviewed.
Data sharing statement All relevant data are within the paper and its supporting materials.